
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
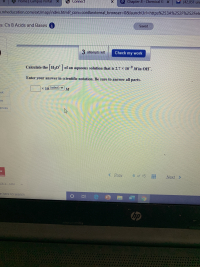
Transcribed Image Text:Calculate the H30" | of an aqueous solution that is 2.7 × 10 M in OH.
Enter your answer in scientific notation. Be sure to answer all parts.
x 10 (select)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Be sure to answer all parts. Hydrofluoric acid, HF, has a K₂ of 6.8 × 10-4. What are [H3O+], [F], and [OH-] in 0.790 MHF? [H3O+] = [ M [f] =[ [OH] = M x 10 M (Enter your answer in scientific notation.)arrow_forwardSolution C: [H3O+]=7.35×10−4 M; [OH−]= Marrow_forwardHello, A solution contains 0.350 grams of malonic acid, H2C3H2O4 in 500.0 mL. (Malonic acid a diprotic acid). What is the % (w/v) of the solution? Thank you,arrow_forward
- Calculate the %(m/v) of hydrochloric acid using the molar concentration you found. Shows calculations. Records final answer to correct significant digits. Records the concentration with the appropriate units. The molar concentration I found was 0.1M.arrow_forwardPlease answer the question! Find the molarity and mass percent of ammonium hydroxide solutions. Thanks!arrow_forwardHello sir, I just want a solution without explanations and a clear linearrow_forward
- Calculate H30" of the following polyprotic acid solution: 0.130 M H2CO3 (Kal 4.3 × 10-7, K2 = 5.6 x 10 11). Express your answer using two significant figures. [H3O*] = Marrow_forwardEach value represents a different aqueous solution at 25 °C. Classify each solution as acidic, basic, or neutral. Acidic Basic Neutral Answer Bank H*] = 2.7 x 10-9 РОН — 4.40 OH| = 4.8 × 109 =D4,8 x РОН 3 13.51 |H*| = 1.0 x 10-7 pH = 5.58 pH = 11.02 H* = 2.9 x 10 %3D pOH = 7.00 OH| = 4.5 x 10-arrow_forwardEach value represents a different aqueous solution at 25 °C. Classify each solution as an acid, base, or neutral. [OH−]= 3.0 × 10 − 6 [H+]= 8.1 × 10 − 13 pOH= 13.67 pOH= 7.00 [H+]= 5.3 × 10 − 3 [OH-]= 3.0x 10^-6 is a(n) Question Blank 1 of 5 , [H+]= 8.1 x 10^-13 is a(n) Question Blank 2 of 5 , pOH= 13.67 is a(n) Question Blank 3 of 5 , pOH= 7.00 is a(n) Question Blank 4 of 5 , [H+]= 5.3 x 10^-3 is a(n) Question Blank 5 of 5arrow_forward
- need help with chemistryarrow_forwardPlease give answer in correct number of sig figsarrow_forwardUsing the constant below, calculate the molar concentration (M) of acid H3O* in solution if the concentration of OH- is 3.4 x 10-5. Kw = 1.0 × 10-14 M²= [H3O*][OH]. %3D O a) 2.9 x 10-10 O b) 2.0 x 10-10 O) 3,4 x 109 d) 2.4 x 10-10 e) 3.4 x 109arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY