
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
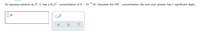
Transcribed Image Text:### Problem Statement
An aqueous solution at 25 °C has a \( \text{H}_3\text{O}^+ \) concentration of \( 8. \times 10^{-6} \, \text{M} \). Calculate the \( \text{OH}^- \) concentration. Be sure your answer has 1 significant digit.
### Diagram
- **Input Box**: A field for entering the answer in molarity (M).
- **Options Below the Input Box**:
- Small checkbox labeled with a multiplication symbol and subscript "x10".
- Two buttons:
- Cancel (×)
- Undo (↻)
- Help (?) button
Note: The diagram is an interactive input field where users submit their calculations and can utilize buttons for actions or assistance.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the solubility of ZnCO, in water at 25 °C. You'll find K data in the ALEKS Data tab. Round your answer to 2 significant digits. g_L x10 X 6arrow_forwardDetermine the concentration of the cation and anion in each aqueous solution. (Assume complete dissociation of each compound.) A. 0.40 MM SrSO4SrSO4 Express your answer using two significant figures. Enter your answers numerically separated by a comma. Sr2+Sr2+, SO42−SO42− = B. 0.10 MM Cr2(SO4)3Cr2(SO4)3 Express your answer using two significant figures. Enter your answers numerically separated by a comma. Cr3+Cr3+, SO2−4SO42− = C. 0.30 MM SrI2SrI2 Express your answer using two significant figures. Enter your answers numerically separated by a comma. Sr2+Sr2+, I−I− =arrow_forward8 pleasearrow_forward
- You are a work study student in our chemistry department. Amy, your supervisor has just asked you to prepare 100ml of 2.50M HClO4 solution for tomorrow's undergraduate experiment. The Stock Solutions cabinet is under the Stockroom Explorer. You will find find a 2.50 liter bottle containing 15M HClO4. Please prepare a flask containing 100ml of a 2.50M solution. To ensure proper credit, please write your calculations in the space given below.arrow_forwardAt 20 °C and a partial pressure of 760 mmHg, the solubility of CO₂ in water is 0.169 g/100 mL. Part A What is the solubility of CO₂ at 2.0×104 mmHg? Express your answer using two significant figures. xa || ΑΣΦ Xb C= 3.38.10³ √x xx x x IXI Submit Previous Answers Request Answer * Incorrect; Try Again; 5 attempts remaining X.10 g/100 mLarrow_forwardAn aqueous solution at 25 °C has a OH concentration of 4. x 10 significant digits. M. Calculate the H,0' concentration. Be sure your answer has the correct number of IMarrow_forward
- 8 + An aqueous solution at 25 °C has a OH concentration of 1. × 10¯³M. Calculate the H₂O* concentration. Be sure your answer has the correct number of significant digits. M X Śarrow_forward1. Draw the organic product (ignoring stereochemistry) 2. State whether the process is overall oxidation, overall reduction, or neither.arrow_forwardHydrochloric acid (11.4 mL of 0.230 M) is added to 279.0 mL of 0.0720 M Ba (OH), solution. What is the concentration of the excess Ht or OH¯ ions left in this solution? Molarity = M Drag and drop your selection from the following list to complete the answer: OH H+arrow_forward
- mL When blood is donated, sodium oxalate solution is used to precipitate Ca2+, which triggers clotting. A 122.0-mL sample of blood contains 9.70 × 10-5 g Ca A technologist treats the sample with 100.0 mL of 0.1550 M Na₂C₂O. Calculate [Ca2+] after the treatment. Be sure your answer has the correct number of significant figures. Note: Reference the Solubility product constants (Ksp) table for additional information. M Ca2+ 5arrow_forwardA student prepares a 3.2M aqueous solution of benzoic acid (C,H,CO,H). Calculate the fraction of benzoic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. | % x10arrow_forwardCalculate the volume in liters of a ×3.210−5m M mercury(II) iodide solution that contains 350.μmol of mercury(II) iodide HgI2 . Be sure your answer has the correct number of significant digits.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY