
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Calculate the enthalpy and entropy of liquid isobutane at T = 160 o F and pressure P = 1000 psia. The following data on specific liquid volume are available in the picture.
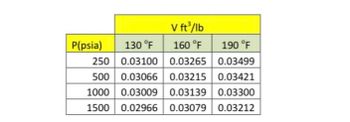
Transcribed Image Text:V ft³/lb
P(psia) 130 °F
160 °F
190 °F
250 0.03100
0.03265
0.03499
500 0.03066 0.03215
0.03215 0.03421
1000 0.03009
0.03139
0.03139 0.03300
1500 0.02966 0.03079 0.03212
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Similar questions
- .30 mol of an ideal gas expands in the diagram. The ideal gas goes through an isochoric heating from point 2 until the pressure isrestored to the value shown in point 1. What is the TF of the ideal gas?arrow_forwardLet’s verify that the macroscopic and microscopic approaches to the calculation of entropy lead to the same conclusion for the adiabatic free expansion of an ideal gas. Suppose the ideal gas as shown expands to four times its initial volume. As we have seen for this process, the initial and final temperatures are the same. (A) Using a macroscopic approach, calculate the entropy change for the gas. (B) Using statistical considerations, calculate the change in entropy for the gas and show that it agrees with the answer you obtained in part (A).arrow_forwardTemperatures (°C) are measured at various points on a heated plate, which are reported in the following table. Estimate the temperature when X=4 and Y=3.2, X=4.3 and Y=2.7 by Lagrange polynomial. Image Help with this problem please, we are working using only X and Y data, but never anything more than this so I don't know how to apply the formulas when we are given more data and I am confused for this task.arrow_forward
- Calculate the total energy, in kilojoules, that is needed to turn a 12 g block of ice at 0 degrees C into water vapor at 135 degrees C.arrow_forwardShow that for any gas whose volume varies linearly with temperature at a given pressure, the Joule–Thomson coefficient is zero.arrow_forwardCalculate the energy associated with a radiation having wavelength 3000 Å. Give the answer in kcal mol-¹ and also in kJ mol-¹.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The