
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:Calculate the amount of heat needed to boil 127. g of benzene (CH₂), beginning from a temperature of 59.1 °C. Round your answer to 3 significant digits.
Also, be sure your answer contains a unit symbol.
x10
X
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
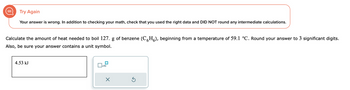
Transcribed Image Text:Try Again
Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations.
Calculate the amount of heat needed to boil 127. g of benzene (CH), beginning from a temperature of 59.1 °C. Round your answer to 3 significant digits.
Also, be sure your answer contains a unit symbol.
4.53 kJ
x10
X
Ś
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question

Transcribed Image Text:Try Again
Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations.
Calculate the amount of heat needed to boil 127. g of benzene (CH), beginning from a temperature of 59.1 °C. Round your answer to 3 significant digits.
Also, be sure your answer contains a unit symbol.
4.53 kJ
x10
X
Ś
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the amount of heat needed to melt 66.7 g of ice (H,O) and bring it to a temperature of 62.2 °C. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol.arrow_forwardA sheet of gold weighing 9.0 g and at a temperature of 13.8 °C is placed flat on a sheet of iron weighing 20.2 g and at a temperature of 57.8 °C. What is the final temperature of the combined metals? Assume that no heat is lost to the surroundings. Be sure your answer has the correct number of significant digits. GC ☐ x10 ☑ ملام 18 Ar 日。 ☑arrow_forwardCalculate the amount of heat needed to boil 45.2g of acetic acid (HCH3CO2), beginning from a temperature of 57.1°C. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol.arrow_forward
- Calculate the amount of heat needed to boil 115. g of acetic acid (HCH,CO,), beginning from a temperature of 33.9 °C. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol. x10arrow_forwardTry Again Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. Calculate the amount of heat needed to melt 130. g of solid acetic acid (HCH³CO₂) and bring it to a temperature of 64.3 °C. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol. 38.2 kJ x10 X Sarrow_forwardGive typed full explanation not a single word hand written otherwise leave itarrow_forward
- A student calibrated a 10-mL graduated cylinder using water. The empty graduated cylinder weighed 24.378 grams. When it was filled with 9.89 mL of deionized water, it weighed 33.826 grams. The temperature of the water was 21.8oC. What is the density of water, based on these measurements?arrow_forwardPlease don't provide handwritten solutionarrow_forwardConstants I Periodic Table Part A How much heat must be absorbed by a 13.0 g sample of water to raise its temperature from 13.0 C to 40.0°C? (For water, C, = 4.18J/g C) Express your answer to three significant figures and include the appropriate units. Value Units Submit Previous Answers Request Answer X Incorrect; Try Again; 29 attempts remaining Provide Feedback acerarrow_forward
- Calculate the amount of heat needed to boil 182. g of benzene (CH), beginning from a temperature of 64.6 °C. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol. 0 x10 Xarrow_forwardA sheet of gold weighing 9,6 g and at a temperature of 16.4 °C is placed flat on a sheet of iron weighing 18.5 g and at a temperature of 50.1 "C. What is the final temperature of the combined metals? Assume that no heat is lost to the surroundings Be sure your answer has the correct number of significant digits. 0arrow_forwardA small piece of iron with a mass of 52.7 grams is heated from 20 degrees Celsius to 31.7 degrees Celsius. How much heat did the iron absorb? The specific heat of iron is 0.450 J/gºC. Remember to round to the nearest 0.01 and include units, capitalized and abbreviated properly.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY