
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Calculate the concentration of lycopene in your orange fraction using using Beer's law equation
A=ExBxC
A is the measured absorbance, E is the molar extinction coefficient (M-1cm-1) at a particular wavelength, B is the path length (in cm) and C is the analyte concentration (M).
A= 0.602. Wavelenght is 500.95nm.
Molar extinction coefficients is 123,000M-1cm-1
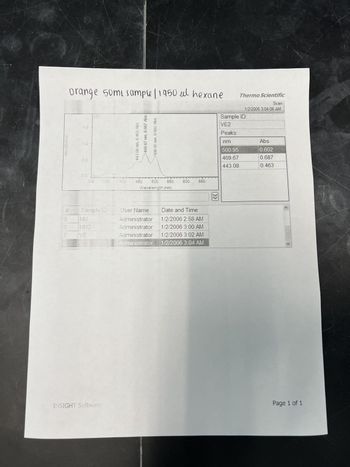
Transcribed Image Text:Absorbance
#
5
6
7
B
orange 50mi sample / 1950 μl hexane
VE2
Sample ID
NM
NM2
INSIGHT Software
Abs
469.67 nm, 0.687 Abs
443.08 nm, 0.463
sqy Z09 0 'wy $6.0093
450 500 550
Wavelength (nm)
600
650
User Name
Administrator
Administrator
Administrator
Date and Time
1/2/2006 2:58 AM
1/2/2006 3:00 AM
1/2/2006 3:02 AM
Administrator 1/2/2006 3:04 AM
Thermo Scientific
Scan
1/2/2006 3:04:06 AM
Sample ID:
VE2
Peaks:
nm
500.95
469.67
443.08
Abs
0.602
0.687
0.463
>
Page 1 of 1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Substance P has an extinction coefficient of 72.22 mM-1cm-1 at 420nm. 2 μl of Substance P was added to 998 μl water and the Absorbance at 420nm was 0.820. Calculate the mM concentration of the original Substance P solutionarrow_forwardReport table 2: Determination of Molar absorptivity of K2CrO4 ppm of K2CrO4 in "DILUTE STOCK (5mL/50mL)"=1.5012 Molarity of K:CrO4 in "DILUTE STOCK(5mL/50mL)" = 7.7319× 10^-3 Peak wavelength λmax=372 nm From single wavelength values Peak Absorbance From spectrum A = 0.487 8=> 1 2 3 4 A= 0.370 A=0.434 A= 0.487 A= 0.347 8= 8= 8= 1.2864×10^6 1.5171×10^6 1.7023×10 6 1.20642×10^6 Average & (for the 4 readings) Standard deviation of a 95% Confidence interval of a = 1.428055×10^6 Show sample calculation (for solution 1): = A/b.c = Molar Absorption= 0,370/(3.72*10^-5)(7.7319× 10^-3)= 1.2864×10^6 M^-1cm^-1arrow_forwardSuppose that a solution has an absorbance of 0.250 at a wavelength of 450 nm. If the concentration of the solution is 22 μM, what is the value of the molar absorptivity? The data were taken with a standard 1-cm cuvette. molar absorptivity in (L cm^−1 mol^-1):arrow_forward
- SPECTROPHOTOMETRY Two groups in a BIO 120 lab extracted a blue-violet pigment from mayana plant which was to be used as a natural dye for Philippine textiles. To determine the concentration of the pigment isolated, each group made a standard curve using commercially available indigo dye in dimethylsulfoxide (DMSO). The following data were obtained by the two groups: Table 4. Absorbance readings of standard solutions of varying concentrations of indigo dye in DMSO. Pure DMSO 0.115 Standard solutions (ppm) Group 1 Data Group 2 Data 0.2 0.398 0.224 0.4 0.563 0.437 0.6 0.732 0.603 0.8 0.833 0.811 1.0 1.008 0.966 1. Show the constructed standard curves of the two groups with proper figure titles. 2. Which of the two groups generated a better standard curve? Justify your answer by providing the R? value for each data set. 3. The extracted pigment has an absorbance of 0.983. When a 0.2 mL-aliquot of the extract was diluted to 1.0 mL, the absorbance of the diluted solution was found to be…arrow_forwardpermanganate solution using the color wheel. ART II: INVESTIGATING ABSORBANCE AND PATHLENGTH 12. Based on Beer's law (A = ɛlC, A = absorbance, ɛ = molar absorptivity, I pathlength and C = concentration), what do you predict is the relationship between pathlength and absorbance? What about pathlength and transmittance?arrow_forwardThe transmitance is 14.4%arrow_forward
- A student mixed 5.00mL of 0.0020M Fe(NO3)3, 3.00mL of 0.0020M KSCN, and 3.00mL of distilled water. The mixture reacted to form a reddish solution indicating that FeSCN2+ was formed, according to the equation below. An absorbance of 0.21 was measured for this solution in a Spectrophotometer set at a wavelength of 447nm. Fe³+ (aq) + SCN (aq) FeSCN2+ (aq) A calibration curve was used to find the equilibrium concentration of the product of this reaction: y = 1895x + 0.027 Calculate the equilibrium concentration of the Fe³+ Report your answer to 4 sig figsarrow_forwardA compound with a molecular weight of 229.61 g/mol was dissolved in 50.0 mL of water. 1.00 mL of this solution was placed in a 10.0 mL flask and diluted to the mark. The absorbance of this diluted solution at 510 nm was 0.472 in a 1.000 cm cuvet. The molar absorptivity of the compound, at 510 nm, is 6,310 M-1 cm-1. Calculate the concentration of the compound in the initial 50.0 mL solution. A. 1.50 x 10-5 M B. 7.48 x 10-5 M C. 7.48 x 10-4 M D. 7.48 x 10-6 Marrow_forwardA calibration curve is made for the absorbance as a function of concentration of blue dye (in mg/L). The slope of the curve is 0.131; the y-intercept is -0.01869; the correlation is 0.993. If a consumer sample has an absorbance of 0.120 on the same instrument, what is its concentration of blue dye, in mg/L? a. 0.034 b. 0.935 c. 0.993 d. 1.06arrow_forward
- Maria was tasked to determine the concentration of a Fe2(SO4)3 solution. She prepared 5 different standards of Fe2(SO4)3 using the table below as her guide. She ran the standards and the unknown solution through UV-VIS spectroscopy and recorded the absorbances of each solution. HINT: use the dilution equation to determine the concentration of Fe2(SO4)3 in each test tube before preparing the standard curve. NOTE: No need to force the line to pass through zero. Just graph the data as is. What is the slope of the line of the standard curve from the given data? What is the concentration of the unknown solution?arrow_forwardAn unknown amount of a compound with a molecular mass of 270.57 g/mol is dissolved in a 10 mL volumetric flask. A 1.00 mL aliquot of this solution is transferred to a 25 mL volumetric flask, and enough water is added to dilute to the mark. The absorbance of this diluted solution at 355 nm is 0.495 in a 1.000 cm cuvette. The molar absorptivity for this compound at 355 nm is €355 = 6149 M-¹cm-¹. What is the concentration of the compound in the cuvette? concentration: What is the concentration of the compound in the 10 ml flask? concentration: How many milligrams of compound were used to make the 10 mL solution? mass: M M mgarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY