
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
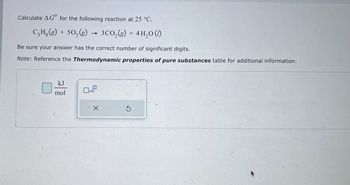
Transcribed Image Text:Calculate AG for the following reaction at 25 °C.
C₂H.(g) + 502(g)
3CO2(g) + 4H2O (1)
Be sure your answer has the correct number of significant digits.
Note: Reference the Thermodynamic properties of pure substances table for additional information.
kJ
mol
Х
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 19 images

Knowledge Booster
Similar questions
- a) Briefly describe the Third Law of Thermodynamics. b) Consider the reaction 2X(g)+ O2(g)→3Y(g) carried out at 25°C and 1 atm. Calculate ΔHrxn°, ΔSrxn°, and ΔGrxn° using the following data. Identify whether the reaction is spontaneous or not. (Assume all gases are perfect). Substance DHfo (kJ/mol) So(J/k mol) X (g) -250 250 Y (g) -300 280 O2 (g) 0 205 c) In a reaction, A + B → Product, rate is doubled when the concentration of B is doubled, and rate increases by a factor of 8 when the concentrations of both the reactants (A and B) are doubled. Choose the correct rate law for the reaction from below. Explain your answer briefly. Rate = k[A][B] Rate = k [A][B]2 Rate = k [A]2[B] Rate = k [A][B]3 d) One mole of N2(g) occupies 5 L of volume at 300 K in a sealed cylinder. The gas behaves ideally in expanding isothermally to 10 L. Calculate w, ΔE and ΔH if the expansion proceeds (i) reversibly, (ii) irreversibly against an external pressure at…arrow_forwardThe standard reaction free energy AG=-1482. kJ for this reaction: 4 Fe(s) + 30₂(g) 2 Fe₂O3(s) Use this information to complete the table below. Round each of your answers to the nearest kJ. reaction 2Fe₂O₂ (s) 4Fe(s) + 30, (g) - Fe(s) + O₂(g) → Fe₂03 (s) Fe₂O₂ (s) → Fe(s) + 0₂ (8) AG kJ kJ X 5arrow_forward5.) Which of the following statements accurately describes the spontaneity of the reaction below?Al2O3(s) + 2Fe(s) → 2Al(s) + Fe2O3(s) H°rxn = 830.8 kJ/mol, S°rxn = 168 J/mol·K a. The reverse reaction is always spontaneous.b. The reaction is spontaneous above 4945 K.c. The reaction is spontaneous below 4945 K.d. The reaction is always spontaneous.e. There is not enough information to determine.arrow_forward
- Consider the reaction described by the chemical equation shown. C,H,(g) + H,O(1) – C,H,OH(1) AH xn = -44.2 kJ Use the data from the table of thermodynamic properties to calculate the value of ASxn at 25.0 °C. ASixn J.K-! Calculate AGixn ·arrow_forwardeq M M IS M ots M M M # 3 E D Consider the reaction 2H₂O(l) 2H₂(g) + O₂(g) The standard free energy change for this reaction is 474.2 kJ. The free energy change when 1.88 moles of H₂O(1) react at standard condition is kJ. 20 C What is the maximum amount of useful work that the reaction of 1.88 moles of H₂O(l) is capable of producing in the surroundings under standard conditions? If no work can be done, enter none. kJ Submit Answer $ 4 R F > % 5 T [References] Use the References to access important values if needed for this question. Retry Entire Group 9 more group attempts remaining G Cengage Learning Cengage Technical Support 6 B MacBook Pro Y Topics] H & 7 N U J ▶II * 00 8 M I ( 9 K. O < 96 ) L P V t Previous Email Instructor Save and Exit { + 11 [ Next ? } 1 delearrow_forward1.) Complete A and B to move on. a.) Write the complete, balanced chemical equation for the following reaction on a separate sheet of paper. Provide products where they are not given. Assume that the reaction is spontaneous. Propane (C3H8) + oxygen → carbon dioxide + water Type the sum of the coefficients in the equation into the answer blank. If there is no coefficient, count it as one. Include ( g, s, l, or aq) b.) Write the complete, balanced chemical equation for the following reaction on a separate sheet of paper. Provide products where they are not given. Assume that the reaction is spontaneous. Diphosphorus pentoxide + water → Type in the coefficient and formula for the product in the equation that contains phosphorus. Include ( g, s, l, or aq)arrow_forward
- Use the data given here to calculate the values of AGan at 25 °C for the Compound AG; (kJ/mol) reaction described by the equation A +387.7 В +596.8 A + B =C +402.0 AGAN kJarrow_forwardA chemical engineer is studying the two reactions shown in the table below. In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 63.0 °C and constant total pressure. Then, he measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of his measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium.arrow_forwardMISSED THIS? Watch KCV 9.10, IWE 9.11; Read Section 9.10. You can click on the Review link to access the section in your eText. Consider the table of Standard Thermodynamic Quantities for Selected Substances at 25 °C. Substance AH (kJ/mol) Substance AH (kJ/mol) Substance AH (kJ/mol) Al(g) HCl(aq) NF3 (g) Al2O3(s) Cr(g) NH3(g) F(g) NH3(aq) C(g) CH4 (g) C₂H₂(g) C₂H4 (8) C₂H6 (g) CO(g) CO₂(g) Cl(g) HCl(g) 330.0 -1675.7 716.7 -74.6 227.4 52.4 -84.68 -110.5 -393.5 121.3 -92.3 HF (g) HF (aq) H(g) Fe(g) FeO(s) Fe2O3(s) Fe3O4(s) N(g) -167.2 396.6 79.38 -273.3 -335.0 218.0 416.3 -272.0 -824.2 -1118.4 472.7 O(g) 03(g) S(g) SO₂(g) SO3(g) Sn(g) SnO(s) SnO₂ (s) -132.1 -45.9 -80.29 249.2 142.7 277.2 -296.8 -395.7 301.2 -280.7 -577.6 Review | Constants | Periodic Table Enter an equation for the formation of CO2 (g) from its elements in their standard states. Express your answer as a chemical equation including phases. C(s) + O2 (g) →CO2 (g) Submit ✓ Correct CO₂ (g) is composed of elements C and O. In…arrow_forward
- Use the given data at 298 K to calculate AG°for the reaction CH4(g) + 2H2S(g) → CS2(g) + 4H2(g) Substance AHof (kJ/mol) S°(J/K·mol) O -50 kJ O 67 kJ O 184 kJ CH4(9) H2S(g) CS2(g) H2(g) -75 186 2.27 x 104 kJ -21 206 90 151 0 131arrow_forwardAt what temperatures will a reaction be spontaneous if AH = +158 kJ and AS = +411 %3D J/K? O The reaction will never be spontaneous. O The reaction will be spontaneous at any temperature. O All temperatures above 384 K O All temperatures below 384 Karrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY