
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
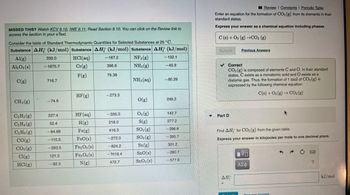
Transcribed Image Text:MISSED THIS? Watch KCV 9.10, IWE 9.11; Read Section 9.10. You can click on the Review link to
access the section in your eText.
Consider the table of Standard Thermodynamic Quantities for Selected Substances at 25 °C.
Substance AH (kJ/mol) Substance AH (kJ/mol) Substance AH (kJ/mol)
Al(g)
HCl(aq)
NF3 (g)
Al2O3(s)
Cr(g)
NH3(g)
F(g)
NH3(aq)
C(g)
CH4 (g)
C₂H₂(g)
C₂H4 (8)
C₂H6 (g)
CO(g)
CO₂(g)
Cl(g)
HCl(g)
330.0
-1675.7
716.7
-74.6
227.4
52.4
-84.68
-110.5
-393.5
121.3
-92.3
HF (g)
HF (aq)
H(g)
Fe(g)
FeO(s)
Fe2O3(s)
Fe3O4(s)
N(g)
-167.2
396.6
79.38
-273.3
-335.0
218.0
416.3
-272.0
-824.2
-1118.4
472.7
O(g)
03(g)
S(g)
SO₂(g)
SO3(g)
Sn(g)
SnO(s)
SnO₂ (s)
-132.1
-45.9
-80.29
249.2
142.7
277.2
-296.8
-395.7
301.2
-280.7
-577.6
Review | Constants | Periodic Table
Enter an equation for the formation of CO2 (g) from its elements in their
standard states.
Express your answer as a chemical equation including phases.
C(s) + O2 (g) →CO2 (g)
Submit
✓ Correct
CO₂ (g) is composed of elements C and O. In their standard
states,
C exists as a monatomic solid and O exists as a
diatomic gas. Thus, the formation of 1 mol of CO₂ (g) is
expressed by the following chemical equation:
C(s) + O2(g) → CO₂(g)
Part D
Previous Answers
Find AH for CO₂ (g) from the given table.
Express your answer in kilojoules per mole to one decimal place.
ΔΗ
V
ΑΣΦ
Request Answer
kJ/mol
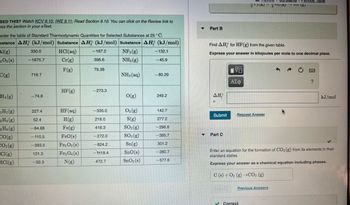
Transcribed Image Text:SED THIS? Watch KCV 9.10, IWE 9.11; Read Section 9.10. You can click on the Review link to
ess the section in your eText.
sider the table of Standard Thermodynamic Quantities for Selected Substances at 25°C.
bstance AH (kJ/mol) Substance AH (kJ/mol) Substance AH (kJ/mol)
HCl(aq)
NF3 (g)
Cr(g)
NH3(g)
F(g)
Al(g)
203 (s)
C(g)
=H₁ (g)
2 H₂(g)
-2 H4 (g)
2 H6 (g)
CO(g)
CO₂(g)
Cl(g)
HCl(g)
330.0
-1675.7
716.7
-74.6
227.4
52.4
-84.68
-110.5
-393.5
121.3
-92.3
HF (g)
HF (aq)
H(g)
Fe(g)
FeO(s)
Fe₂O3(s)
Fe3O4(s)
N(g)
-167.2
396.6
79.38
-273.3
-335.0
218.0
416.3
-272.0
-824.2
-M18.4
472.7
NH3(aq)
O(g)
03 (g)
S(g)
SO₂(g)
SO3(g)
Sn(g)
SnO(s)
SnO₂ (s)
-132.1
-45.9
-80.29
249.2
142.7
277.2
-296.8
-395.7
301.2
-280.7
-577.6
▼
Part B
ΔΗ
Submit
Find AH for HF (g) from the given table.
Express your answer in kilojoules per mole to one decimal place.
Part C
V
ΑΣΦ
Request Answer
Constants
22(6/2¹22\5/11 (5)
Periodic Table
Previous Answers
✓ Correct
?
Enter an equation for the formation of CO₂ (g) from its elements in their
standard states.
Express your answer as a chemical equation including phases.
C(s) + O₂ (g) →CO₂ (g)
kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3.) Consider the reaction below. Reaction: AH°r (kJ/mole) 2 ICI (£) 17.78 L. (g) 247.551 l16.135 223.066 (/mole K) Which explains the outcome when 0.45 mol ½, 0.45 mol Cl2, and 2.42 mol ICl are added to a 2.0L flask at 25°C? A. [ICI] decreases B. [ICI] increases C. both [b] and [CL] increase D. at equilibrium Keywords: reaction quotient (Q), initial concentration(s), equilibrium concentration(s), equilibrium constant, forward reaction, reverse reaction, Q K. Q= K Concepts: Reaction Quotient, Chemical equilibriumarrow_forwardCalculate the standard free energy change (kJ/mol) for the reaction shown at a temperature of 298.15 K using the information provided. Reaction ΔHrxn (kJ/mol) ΔSrxn (J/mol-K) 2 Mn(s) + 3 O2(g) → 2 Mn2O3(s) -1917.9 -458.7arrow_forwardΣ *00 For a particular reaction, AH = 81.95 kJ and AS = 27.0 J/K. Calculate AG for this reaction at 298 K. AG = %3D What can be said about the spontaneity of the reaction at 298 K? O The system is spontaneous in the reverse direction. O The system is at equilibrium. O The system is spontaneous as written. 8:12 PH (口 四< 11/8/20 F5 F8 F12 PrtScr Insert Delete Backspace $4 % 8. 5. 7. 24 Alt Ctriarrow_forward
- eq M M IS M ots M M M # 3 E D Consider the reaction 2H₂O(l) 2H₂(g) + O₂(g) The standard free energy change for this reaction is 474.2 kJ. The free energy change when 1.88 moles of H₂O(1) react at standard condition is kJ. 20 C What is the maximum amount of useful work that the reaction of 1.88 moles of H₂O(l) is capable of producing in the surroundings under standard conditions? If no work can be done, enter none. kJ Submit Answer $ 4 R F > % 5 T [References] Use the References to access important values if needed for this question. Retry Entire Group 9 more group attempts remaining G Cengage Learning Cengage Technical Support 6 B MacBook Pro Y Topics] H & 7 N U J ▶II * 00 8 M I ( 9 K. O < 96 ) L P V t Previous Email Instructor Save and Exit { + 11 [ Next ? } 1 delearrow_forwardA chemist fills a reaction vessel with 2.90 atm nitrogen monoxide (NO) gas, 6.95 atm chlorine (Cl,) gas, and 7.94 atm nitrosyl chloride (NOCI) gas at a temperature of 25.0°C. Under these conditions, calculate the reaction free energy AG for the following chemical reaction: 2NO (g) + Cl, (g) 2NOC1 (g) Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule.arrow_forwardUsing enthalpies of formation, calculate deltaH for the following reaction at 25°C. Also calculate deltaS for this reaction from standard entropies at 25°C. Use these values to calculate deltaG for the reaction at this temperature. Species deltaHf(kJ/mol) S(J/mol⋅K) CH3OH(l) –238.7 126.8 O2(g) 0 205.0 CO2(g) –393.5 213.7 H2O(l) –285.8 69.95 2CH3OH(l) +3O2(g) --- 2CO2(g) + 4H2O(l) deltaH = kJ deltaS = J/K deltaG = kJarrow_forward
- For each of the following reactions, calculate ΔH∘rxn, ΔS∘rxn, and ΔG∘rxn at 25 ∘C. State whether or not the reaction is spontaneous. If the reaction is not spontaneous, would a change in temperature make it spontaneous? If so, should the temperature be raised or lowered from 25 ∘C?2CH4(g)→C2H6(g)+H2(g), Calculate ΔS∘rxn at 25 ∘C. 2NH3(g)→N2H4(g)+H2(g) Calculate ΔS∘rxn at 25 ∘C. N2(g)+O2(g)→2NO(g) Calculate ΔS∘rxn at 25 ∘C. 2KClO3(s)→2KCl(s)+3O2(g) Calculate ΔS∘rxn at 25 ∘C.arrow_forwardAGO SnO₂(s) + 2 CO(g) → Sn(s) + 2 CO₂(g) Use this information to complete the table below. Round each of your answers to the nearest kJ. The standard reaction free energy reaction 2Sn (s) + 4CO₂(g) == - 194. kJ for this reaction: 2SnO₂ (s) + 4CO(g) 3 SnO₂ (s) + 6CO(g) → 3Sn(s) + 6CO₂(g) Sn(s) + 2CO₂(g) SnO₂ (s) + 2CO (g) AGⓇ ☐ kJ kJ KJ x10 X Śarrow_forward65arrow_forward
- A chemist fills a reaction vessel with 1.75 atm nitrogen monoxide (NO) gas, 1.94 atm chlorine (Cl₂) gas, and 4.19 atm nitrosyl chloride (NOCI) gas at a temperature of 25.0°C. Under these conditions, calculate the reaction free energy AG for the following chemical reaction: 2NO(g)+Cl₂(g) 2NOCI(g) Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule.arrow_forwardAnswer this question without using numbers from the book (or anywhere else!) ΔS for the following reaction is negative. True or false? 2 CO(g) + O2(g) → 2 CO2(g)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY