
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
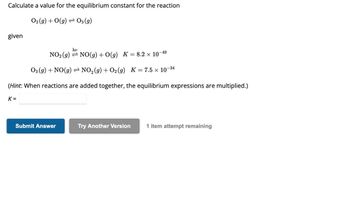
Transcribed Image Text:Calculate a value for the equilibrium constant for the reaction
O₂(g) + O(g) → 03 (g)
given
hv
-49
NO₂ (g) NO(g) + O(g) K = 8.2 × 10¯
O3(g) + NO(g) ⇒ NO₂(g) + O₂(g) K = 7.5 × 10-
-34
(Hint: When reactions are added together, the equilibrium expressions are multiplied.)
K=
Submit Answer
Try Another Version 1 item attempt remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the equilibrium constant for this reaction given the following information. CO (g) + 2 H2S (g) CS2 (g) + H2O (g) + H2 (g) K =? = 5.49 · 102 CH. (g) + H20 (g) → CO (g) + 3 H2 (g) K CHA g) + 2 H2S (g) CS2 (g) + 4 H2 (g) K = 2.70 . 105 2.03 10-3 4.92 102 3.03 103 O 1.48 · 10° « Previous MacBook Air 80 888 DII F3 F4 F5 F6 F7 F8 F9 23 $ & 3 4 6. 7 8. E R Y U LIarrow_forwardSo Please don't provide the handwriting solutionarrow_forwardUsing the equilibrium constants given for reactions (1) and (2), what is the equilibrium constant for reaction (3)? 1) 2NO(g) + O2(g) → N2O4(g) Kp = 1.49 × 1013 2) 2NO(g) + O2(g) → 2NO2(g) Kp = 1.66 × 1012 3) N2O4(g) → 2NO2(g) 80.3 0.111 None of the following 8.98 0.0123arrow_forward
- Given the folowing equilibria: cog) + 2 H2(g) = CH;OH(g) H20(g) + C(s) = H2(g) + Co(g) K= 10. x 10-5 K= 6.0 x 10-5 What is the equilibrium constant for the following reaction? C(s) + H2(g) + H,0(g) = CH;OH(g) K = ? A. 1.6 x 104 В. 6.0 x 10-5 C. 1.6 x 10-9 D. 6.0 x 10-10 Е. 6.0 x 10-9arrow_forwardWrite the equilibrium constant expression, K, for the following reaction: (If either the numerator or denominator is blank, please enter 1.) COC12 (g) CO(g) + Cl₂ (g) K =arrow_forward3. If the equilibrium constant K, for the reaction 2 NO (g) + O2(g) = 2 NO2 (g) is 5.0 x 1012 at a given temperature, what is the value of the equilibrium constant K, for each of the following reactions at the same temperature? a) NO (g) + ½ O2 (g) → NO2 (g) b) 2 NO2 (g) = 2 NO (g) + 02 (g) c) NO2(g) = NO (g) + ½ 02 (g)arrow_forward
- At a particular temperature, K = 1.00 x 10² for the reaction H₂(g) +1₂(g) → 2HI(g) In an experiment, 1.00 mol H₂, 1.00 mol 12, and 1.00 mol HI are introduced into a 1.00 L container. Calculate the equilibrium concentrations of all reactants and products.arrow_forwardThe equilibrium constant, Kc, is given for one of the reactions below. What is the value of the missing equilibrium constant, Kc? Cl₂(g) + H₂O(g) = 2 HCl(g) + 1/2 O₂(g) K = 7.52 × 10-2 4 HCI(g) + O₂(g) = 2 Cl₂(g) + 2 H₂O(g) OK= 5.66 x 10-3 OK= 13.3 OK= 0.150 OK = 177 OK = 3.65 K = ?arrow_forwardThe equilibrium constant (K1) for the reaction CO2 (g) 2 CO(g) +1/2 02(8) is 6.66 x 10-12 at 1000 K. Calculate K2 for the reaction 2 CO(g) + 02(g) 2 2002(g) K2 =arrow_forward
- 10. What is the expression for the equilibrium constant (K) for the reaction 2 C₂H(g) +7 O2(g) → 4 CO2(g) + 6 H₂O(g)? Tool Box: 1 cal 4.184 J 1Kcal 4.184 KJ 1000 cal 1 Kcal 1000 J = 1 KJarrow_forwardUse the References to access important values if needed for this question. Consider the reaction: C(s) + 1/20₂(g) → CO(g) Write the equilibrium constant for this reaction in terms of the equilibrium constants, K₁ and K2, for the reactions below: C(s) + O2(g) CO₂(g) K₁ CO(g) + 1/2O₂(g) = CO2 (9) K₂ For answers with both a subscript and a superscript, enter the subscript first. For example, enter Kif the first equilibrium constant should be squared. K =arrow_forwardPhosgene is a chemical warfare agent first used on soldiers in World War I. It is synthesized according to the following gas-phase equilibrium reaction: CO(g) + Cl, (g) = COCI,(g) Write the equilibrium expression K, for this reaction below. K. = If K. = 0.281 for this reaction, would the product or the reactant be favored at equilibrium? The reactants would be favored. Neither the product nor the reactants would be favored. The product would be favored.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY