
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
only need the hybridization and bond angle of d-f.
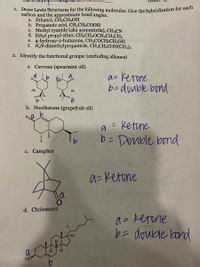
Transcribed Image Text:1. Draw Lewis Structures for the following molecules. Give the hybridization for each
carbon and the approximate bond angles.
a. Ethanol, CH3CH2OH
b. Propanoic acid, CH3CH2COOH
c. Methyl cyanide (aka acetonitrile), CH3CN
d. Ethyl propyl ether, CH3CH2OCH2CH2CH3
e. 4-hydroxy-2-butanone, CH3COCH2CH2OH
f. N,N-dimethylpropamide, CH3CH2CON(CH3)2
2. Identify the functional groups: (excluding alkanes)
a. Carvone (spearmint oil)
a= Ketone
b-double bond
(R)
(S)
b. Nootkatone (grapefruit oil)
= Ketone
b= Donble bond
%3D
c. Camphor
a= ketone
monetiv
OH
d. Cholesterol
HO
a = ketone
%3D
b= doubde land
..
H
a
HO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider a 2s orbital and a 2p orbital that come hybridize to form two sp orbitals. Which of the following statements is true?arrow_forwardIdentify hybridization and bond anglearrow_forward3. Identify the hybridization and the approximate bond angles around each atom marked with an arrow in the molecule nepetalactone, one of several compounds in catnip known to induce cat "friskiness." geometry shape hybridization bond angles geometry shape hybridization bond angles nepetalactonearrow_forward
- 4.) Fill in the following table with the number of carbon atoms of the specific hybridization state belonging to the following molecule. CH3 CN Hybridization states of carbon # of carbon atoms sp sp? sparrow_forwardAssign hybridization state of each carbon, nitrogen, oxygen, halogen, and boron atom indicated with arrows in the molecules below.arrow_forwardNiteshharrow_forward
- The percentage p-character in sp hybridized orbitals is a) 25% b) 50% c) 75% d) 66.67%arrow_forward2) a) Consider the following molecule . Given what you have learned about hybridization theory, draw an image or images explaining the bonding situation in this molecule. I want you to draw out all of the orbitals, hybrid orbitals and how they overlap to form the bonds in the molecule. Indicate the % s or p character in the given atomic and hybrid orbitals. Which C-C bond or bonds are the longest? In a paragraph or so explain the image or images you just drew. b) Lastly, consider the molecule below. Indicate the Molecular formula, the molar mass, label the hybridization of each atom except for hydrogen, indicate any chiral centers with a *, which bond or bonds are the shortest, identify by name of each functional group with an arrow pointing to the group.arrow_forward16arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY