
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Transcribed Image Text:C
www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-IQUHIQg6bJxmeSyVPHOEB1plef9xyC5Ca9QIn2ZR7SUf70J1_Z3x40AgDxMQTd47AtHiXijWvKqzP7Uj...
Naming and Drawing Organic Molecules
Recognizing different skeletal structures
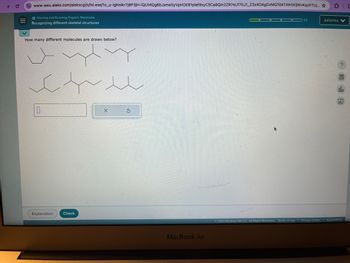
How many different molecules are drawn below?
Explanation
Check
X
G
MacBook Air
1/5
Julianna V
?
ala
Ar
2024 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- R lawarrow_forwardas X Clas X ||| STE X © Ban X Jen Scho × Ban X CD www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNsikr7j8P3jH-liG_IZvpRqwiHv-fgOzocXR7H3QULJsrn-hH7iM_OKtS081EM1kmGkq29c dent Bookmarks 00 Duolingo - The worl... ▸ YouTube Mary G. Ross: Who... Zomberry Hero > Try Again Cha X O MATTER Finding the side length of a cube from its volume in liters Your answer is incorrect. 0.75 m Trial X Explanation A technical machinist is asked to build a cubical steel tank that will hold 475 L of water. Recheck 198 X Calculate in meters the smallest possible inside length of the tank. Round your answer to the nearest 0.01 m. → # X $ " % d H 6 H Copy of Cause and... FORENSIC SCIENC... kh hp Bay x M & 7 Ⓒ2023 McGraw Hill LLC. All Rights Re C Cop X * 00 8 ( 9arrow_forward● A ALEKS-Lara AX → с www-awu.aleks.com/alekscgi/x/lsl.exe/10_u-IgNslkr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHIntrzjVfiaJ_cKvcyqWTUEIAKXtcoqhtE9_vix... 4 Solubility and... 18.3 Gibbs Free E... 5.3 Enthalpies of.... 18.5 Gibbs Free E... Reading Schedule 19.6 Reduction Po... C ! 1 Under these conditions, calculate the reaction free energy AG for the following chemical reaction: N₂(g) + 3H₂(g)2NH₂(g) Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule. ● ENTROPY AND FREE ENERGY Calculating reaction free energy under nonstandard conditions Explanation A T A chemist fills a reaction vessel with 7.76 atm nitrogen (N₂) gas, 7.51 atm hydrogen (H₂) gas, and 0.524 atm ammonia (NH₂) gas at a temperature of 25.0°C N Puppy Jobs, Em x G puppy store ne x Part Time Jobs, x G Food Server and X G 10 out of 25 as x) How Many Hour x CO @ 2 Check W S X R option command #3 X E D 26 $ DS 4 C Ś R LL F % 5 V T tv SH N G MacBook Pro ^ 6 Y B & M 7 19.6 Reduction…arrow_forward
- MALEKS T LI ALEKS - Rafia Riaz - Knowledge CX + www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusWtHPKeXbFh_esrw9lm-BbWISQqFfd1OKKzQ55nmjVf-6W1kBQ5aycVAYBaQo?... Type here to search = Module Knowledge Check Read the descriptions of physical or chemical changes in the table below. Then decide whether the change will be spontaneous, if you can. A chemical reaction between two liquids creates gaseous products. A gas expands, absorbing heat from its surroundings. Change During an exothermic chemical reaction, a solid is consumed and a gas produced. I Don't Know Et Submit Question 1 3 Is this change spontaneous? Yes. O No. O Can't decide with information given. O Yes. O No. O Can't decide with information given. O Yes. O No. O Can't decide with information given. X Rafia V ? 13 ollo © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 65°F Mostly clear (?) F * (P 9:08 PM 5/10/2023 x : 6arrow_forwardA ALEKS-Lara Althawadi O ☆ www-awu.aleks.com/alekscgi/x/isl.exe/10_u-IgNsikr7j8P3JH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHInCwZsOvg6oljUPZmqCsVfxljXe3yeB7wt2E... a Solubility and... 18.3 Gibbs Free E... 5.3 Enthalpies of.... 18.5 Gibbs Free E... Reading Schedule 19.6 Reduction Po... Y SOLUTION: The le... Math 115 W-S Fall.... h. Get star O ACIDS AND BASES Calculating the pH at equivalence of a titration pH = 0 A chemist titrates 120.0 mL of a 0.8655 M ammonia (NH3) solution with 0.7463M HBr solution at 25 °C. Calculate the pH at equivalence. The pK, of ammonia is 4.75. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. Explanation Check ethods for Measuring the pH of an Aqueous 28 of 104 Notes 2 x S Comments Click to add notes 3 E X :: D $ 4 C Q S > R F 5 I T + 63% G 6 53 B I'm Y 7 H Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |…arrow_forwardCan you please help with this question I have attached a picture of. Thank you so much!arrow_forward
- M Gmail T ALEKS - Rafia Riaz - Learn Significant Figures Calculator - Six b Home | bartleby www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusCO2wDIf49ytsp8EECIGBi6veibDgHNs16W4StInReRBanAJb03txg-0axcq?1oBw7QYjlbavbS... ☆ YouTube Maps X C Calculate the amount of heat nee X Type here to search Translate Explanation News O STATES OF MATTER Using heat of fusion or vaporization to find the heat needed to... College information Check Calculate the amount of heat needed to melt 141. g of ice (H₂O) and bring it to a temperature of 54.1 °C. Be sure your answer has a unit symbol and the correct number of significant digits. 0 발 x10 X G Calculate the amount of heat nee X 9 1/3 + © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Earnings upcoming (32) Rafia V olo Ar Accessibility J ★ x Other bookmarks 10:52 PM 2/8/2023arrow_forwardMALEKS T LI ALEKS - Rafia Riaz - Knowledge CX + www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusWtHPKeXbFh_esrw9lm-BbWISQqFfd1OKKzQ55nmjVf-6W1kBQ5aycVAYBaQo?... Type here to search = Module Knowledge Check A chemical engineer is studying the two reactions shown in the table below. In each case, she fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 33.0 °C and constant total pressure. Then, she measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of her measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor…arrow_forwardHelp 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forward
- °F rtly cloudy m/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-lvWyv8WYLP6W0cqJcWJdIACROQwyw24GWHInMM72ts 1 NTBcSzwZOGGhrmIP4yCejeRhX9HIzqlzRTj2iaDBXTCRIHYWNO_-o?10... O CHEMICAL REACTIONS Calculating molarity using solute moles A chemist prepares a solution of mercury(II) iodide (HgI₂) by measuring out 0.0161 μmol of mercury(II) iodide into a 300. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in μmol/L of the chemist's mercury(II) iodide solution. Round your answer to 3 significant digits. µ mol L Explanation Check x10 X Ś Q Search 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use **************** D 1/5 www Jessi Privacy Center | Accessibilityarrow_forwardRb Blackboard Collaborate Ultra -2 x General Psychology -Fall 20 How to Find a Career Path Using X V What Kind of Intelligence Do You X A ALEKS - Griffin Barden- Learn com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IJcZzdcvSCzsqTCIDqNGV3bKqhMfPmUcQ4ENkmiXn9QCwgeDPDkQ06yszYWESPcekwL0-Qg6l19rekU7404HgFAGBEZaDr080?1oBw7QYjlbavbSPXtx-YCjsh_7mMmrq O THERMOCHEMISTRY Griffin Calculating a molar heat of reaction from formation enthalpies Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard conditions: (6)°Hɔ-(5)arrow_forwardGven: 10mL of Hd Dotll= mass of NaOH2.013a MHC- 1.0 OM M Naot-1.0OM NaOt(s)+HCI CaqNaulaa+H2O) Atrin J/molarrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY