
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
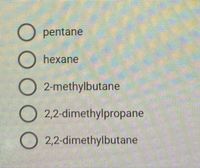
Transcribed Image Text:The image shows a list of chemical compounds, each preceded by a radio button, suggesting it is part of a multiple-choice question. The compounds listed are:
1. Pentane
2. Hexane
3. 2-Methylbutane
4. 2,2-Dimethylpropane
5. 2,2-Dimethylbutane
There are no graphs or diagrams included in the image.
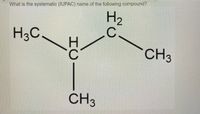
Transcribed Image Text:**Title: Understanding the Systematic (IUPAC) Naming of Alkanes**
**Description:**
The image presents a structural formula of an organic compound, which is a part of the alkane family. The task is to determine the systematic (IUPAC) name for this compound.
**Molecular Structure Details:**
1. **Central Carbon Atom Arrangement:**
- The structure consists of a central carbon atom bonded to three other carbon atoms.
2. **Alkyl Groups:**
- One of these carbon atoms is bonded to two hydrogen atoms (CH₃), representing a methyl group.
- Another carbon atom is bonded to two hydrogen atoms (CH₂).
- The third carbon atom, also a CH₃ group, is attached directly to the central carbon atom.
3. **Chemical Structure Explanation:**
- The compound includes four carbon atoms in total.
- The main carbon chain has three carbon atoms with a single methyl group branching off the second carbon.
**IUPAC Naming Convention:**
Using IUPAC nomenclature rules for alkanes, the longest carbon chain is identified and named, and substituents are assigned numbers based on their position along the chain:
- The longest continuous chain of carbon atoms consists of three carbons, named "propane."
- There is a methyl group (CH₃) attached to the second carbon of the propane chain.
Hence, the compound is named:
**2-Methylpropane**
This name indicates a propane chain with a methyl group substitution at the second carbon. This is also commonly known as isobutane.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY