Question
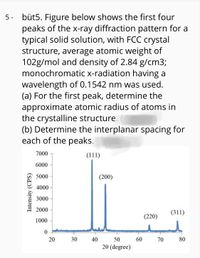
Transcribed Image Text:5 - büt5. Figure below shows the first four
peaks of the x-ray diffraction pattern for a
typical solid solution, with FCC crystal
structure, average atomic weight of
102g/mol and density of 2.84 g/cm3;
monochromatic x-radiation having a
wavelength of 0.1542 nm was used.
(a) For the first peak, determine the
approximate atomic radius of atoms in
the crystalline structure.
(b) Determine the interplanar spacing for
each of the peaks.
7000
(111)
6000
5000
(200)
4000
3000
2000
(311)
(220)
1000
20
30
40
50
60
70 80
20 (degree)
Intensity (CPS)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Similar questions
- A mode-locked laser has a linewidth of 100nm at a center wavelength 1050nm. Assume the pulse shape is hyberbolic secant and it is transform limited. What is the pulse width (in fs)? (round all answers to 1 sig. fig. after decimal point) a.116.0 b.11.6 c.5.8 d.58.0 e.100.0arrow_forwardPotassium iodide has an interplanar spacing of d = 0.296 nm. A monochromatic X-ray beam shows a first-order diffraction maximum when the grazing angle is 7.4°. Calculate the X-ray wavelength ?.? = answer in nmarrow_forward1) A laser has divergence of 1 milli radians . What is the spatial spread for every meter ? The answer is 1 mm . Please explain how the answer is got 2) You are given a light source of coherence length 3 m . It is split into 2 rays such that the path difference between them is 4 m . Can you form a stable interference ? The answer is No . I just want the reasoning to the answer .arrow_forward