
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Propose the synthesis of the following molecule from any organic solvents or reagent, given that acetylene is only source of carbon.
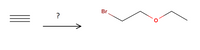
Transcribed Image Text:The image depicts a chemical reaction scheme.
**Left Part:**
- Three stacked lines (≡) represent a triple bond, suggesting the presence of an alkyne group, such as an acetylene (C≡C).
- There is a question mark ('?') beneath the alkyne, indicating uncertainty or the need to determine the conditions or reagents necessary for the reaction.
**Arrow:**
- A rightward-pointing arrow signifies the progression of the chemical reaction.
**Right Part:**
- The product is a molecular structure composed of:
- A bromine atom (Br) attached to a three-carbon chain.
- There is also an ether linkage, with an oxygen atom (O) connecting the third carbon atom to a two-carbon chain.
This image represents a reaction involving an alkyne and the formation of a bromo ether, which could involve transformations such as hydroboration-oxidation or the addition of a halogen.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Propose a multi-step synthetic route to form the polycyclic molecule on the right hand side using the following reagents on the left hand side of the arrows: i) cyclic ketone; ii) a,ẞ-unsaturated ketone; iii) either the organocuprate OR the organolithium reagent listed; and ii) any other reagents or solvents of your choosing The depicted reagents represent all necessary building blocks that are directly incorporated into the molecule on the right, however you should use additional reagents to help facilitate its construction based on Chem 110 content from Chapters 21 and 23. This is a multi-step synthesis and not all reagents will be used at the same time. When describing your solution, show the stable intermediates along your proposed synthetic route and number the individual synthetic steps. You do not have to worry about stereochemistry in this particular question. For two (2) of your proposed steps, show individual mechanistic arrows that show the flow of electrons during that…arrow_forwardProvide the reagents necessary to complete the following synthesis. ?arrow_forwardHow would I produce ethoxybenzene from benzenearrow_forward
- From naphthalene and any other organic or inorganic reagent synthesize the following molecule.arrow_forwardThe academic version of the process invention program (PIP) contains several examples, as follows: HDAl-hydrodealkylation of toluene to produce benzene CYHEXl-cyclohexane production by hydrogenation of benzene STYRl-styrene production from ethylbenzene XYLl-production of m-xylene from toluene ANHYDl-reaction of acetone and acetic acid to form acetic anhydride BUTALl-alkylation reaction of butene-1 and isobutane to produce iso-octane Select the “new plant design” mode and the “look at existing flow sheet” option, and follow the synthesis steps for one of these processes. ‘Obtain the current flow sheet as output at each level of the synthesis procedure. Also list the heuristics used at each step.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY