
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
write the structure for products.(M,N,O)
ONLY TYPED SOLUTION
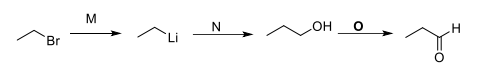
Transcribed Image Text:M
N
OH
°
H
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When the following equation is balanced properly under basic conditions, what are the coefficients of the species shown? 2- so,2- + so,?- + N2H4 Water appears in the balanced equation as a (reactant, product, neither) with a coefficient of (Enter 0 for neither.) How many electrons are transferred in this reaction? Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardPredict Products. Using line structures, give the major product of each 1.[ reaction below. DON'T SHOW MECHANISMS HERE. Use scratch paper if needed. H2 a) Pt/C b) HBr H2SO4 HOarrow_forward
- 3. (a) Draw the appropriate curved arrows and products for each set of reactants undergoing a coordination step. Identify each reactant species as either a Lewis acid or a Lewis base. (b) Use curved arrow notation to show each product undergoing heterolysis to regenerate reactants. (0) (ii) NH₂ H₂O + BF3 + AICI3arrow_forwardGive detailed Solution with explanation neededarrow_forwardGive correct detailed Solution with explanation needed..don't give Handwritten answerarrow_forward
- [References] Use the References to access important values if needed for this question. Draw the structure of the organic product that is expected when the following compound is treated with concentrated H,SO4. CH3 CH2CCH2CH3 tosH heat HO. • You do not have to consider stereochemistry. • In cases where there is more than one answer, just draw one. ed P. opy aste C. CH4 ChemDoodle Retry Entire Group 3 more group attempts remaining Submit Answer Previous Next Email Instructor Save andarrow_forwardpease,don't provied handwriting solution.arrow_forwardFor the reaction in the image attached, write a mechanism, include curved arrows showing bonding changes.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY