
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Which of the following structures represent the same stereoisomer?
a) only I and II
b)only II and III
c) I,II, and III
d) only I and III
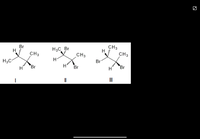
Transcribed Image Text:The image displays three different stereochemical representations of molecules, each depicting various spatial arrangements of atoms around a central carbon atom:
- **Molecule I**: Shows a central carbon atom bonded to a hydrogen atom (H), a bromine atom (Br), and two methyl groups (CH₃). The bonds are shown in different spatial orientations using wedge (coming out) and dash (going in) notation.
- **Molecule II**: Similar to Molecule I, this structure also has a central carbon atom bonded to a hydrogen atom, a bromine atom, and two methyl groups. The spatial arrangement differs from Molecule I, indicated by the orientation of the wedge and dash bonds.
- **Molecule III**: This structure features a similar set of bonds as the previous molecules but with another unique spatial arrangement. The configuration of the wedge and dash bonds is distinct from those in Molecules I and II.
These diagrams illustrate different stereoisomers, where compounds have the same molecular formula but differ in the three-dimensional arrangement of atoms. Understanding stereochemistry is crucial for recognizing how molecular structure influences chemical behavior and interactions.
Expert Solution
arrow_forward
Step 1
Stereoismers have identical molecular formula and arrangement of atoms.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 1.1 Which of the following statements is true for the chemical depicted below? CH,-CH,-CH,-CH,-CH2-CH,-CH,-CH,-CH2-CH2-CH,-CH3 CH3-N-CH3 CH,-CH,-CH,-CH,-CH2-CH,-CH,-CH,-CH,-CH,-CH,-CH3 A) it has unsaturated hydrocarbon chains B) this molecule is a cationic lipid c) this molecule readily dissolves in water D) this molecule could be useful for carrying nucleic acids into cellsarrow_forwardHaving peptides arranged in a beta sheet would be an example of a secondary structure A) True B) Falsearrow_forwardThe structures below are two different _______________ . a) Conformational Isomers b) Stereoisomers c) Constitutional Isomers d) Views of the same moleculearrow_forward
- What does the structure in yellow do?arrow_forward38) A nucleic acid sequence is read from the A) free 3' hydroxyl; free 5' phosphate B) free 3' phosphate; free 5' hydroxyl C) free 5' phosphate; free 3' hydroxyl D) free 5' hydroxyl; free 3' phosphate to thearrow_forwardUsing the appropriate chemical structures describe the monomers and polymers for each of the following macromolecules; a) proteins b) carbohydratesarrow_forward
- A) B) A. Name specific structure "A" B. Name specific structure "B"arrow_forwardHypoglycin A, an amino acid derivative found in unripened lychee, is an acutely toxic compound that produces seizures, coma, and sometimes death in undernourished children when ingested on an empty stomach. (a) Draw the neutral, positively charged, and negatively charged forms of hypoglycin A. (b) Which form predominates at pH = 1, 6, and 11? (c) What is the structure of hypoclycin A at its isoelectric point?arrow_forwardRank the following three compounds in order of decreasing basicity. NH2 NH₂ NH2 NO2 I II III A) II > I > III B) III II I C) III > I > II D) I > II > IIIarrow_forward
- I was at the supermarket the other day shopping for groceries, and I picked up a box of Schtuck Margarine. On the front of the box, it listed “5% trans fats” and on the ingredient list it had “hydrogenated corn oil” listed as the main ingredient. If corn oil is a polyunsaturated vegetable oil with cis- double bonds (only), why is it hydrogenated, and where are the trans fats coming from? Additionally, what is the issue with trans- fats?arrow_forwardFor the following molecule, I need to identify several things, but I don't understand how to go about it. a) anomeric carbon b) carbon 1 c) carbon 5 d) which oxygen atoms in a hydroxyl group would point to the right in a Fischer Projectionarrow_forwardA) what does the figure illustrate? B) Label the components in the figure pointed by the arrows and briefly mention the bonding partners involved in each casearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON