
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
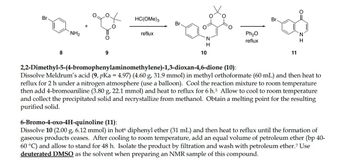
Please help me calculate the theoretical yield(s) for this reaction as seen in the photo! All necessary information can be found there.
Transcribed Image Text:Br
8
NH₂
9
HC(OME) 3
reflux
Br.
H
10
Ph₂O
reflux
Br.
11
H
2,2-Dimethyl-5-(4-bromophenylaminomethylene)-1,3-dioxan-4,6-dione (10):
Dissolve Meldrum's acid (9, pKa = 4.97) (4.60 g, 31.9 mmol) in methyl orthoformate (60 mL) and then heat to
reflux for 2 h under a nitrogen atmosphere (use a balloon). Cool the reaction mixture to room temperature
then add 4-bromoaniline (3.80 g, 22.1 mmol) and heat to reflux for 6 h.5 Allow to cool to room temperature
and collect the precipitated solid and recrystallize from methanol. Obtain a melting point for the resulting
purified solid.
6-Bromo-4-oxo-4H-quinoline (11):
Dissolve 10 (2.00 g, 6.12 mmol) in hot diphenyl ether (31 mL) and then heat to reflux until the formation of
gaseous products ceases. After cooling to room temperature, add an equal volume of petroleum ether (bp 40-
60 °C) and allow to stand for 48 h. Isolate the product by filtration and wash with petroleum ether.7 Use
deuterated DMSO as the solvent when preparing an NMR sample of this compound.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- [Tutorial: Limiting reactant stoichiometry] This question will walk you through the steps of calculating the mass of products produced based on your determination of the limiting reactant. b) Step 2a: Use dimensional analysis to determine the theoretical yield of the product. Calculate the theoretical yield in grams Al₂O₃ from the complete reaction of 64.7 grams Al according to the following balanced chemical equation: 2 Al(s) + Fe₂O₃(s) → Al₂O₃(s) + 2 Fe(s) c) Calculate the theoretical yield in grams Al₂O₃ from the complete reaction of 201 grams Fe₂O₃ according to the following balanced chemical equation: 2 Al(s) + Fe₂O₃(s) → Al₂O₃(s) + 2 Fe(s) d) Which of the following substances is the limiting reactant? e) What is the mass in grams of the excess Fe₂O₃ remaining after the partial reaction of 201 g Fe₂O₃ with 64.7 g Al? Give your answer to three significant figures.arrow_forwardThe actual yield of one of the reactions in 43.5g. What was theoretical yield in the laboratory if the scientist concluded that % yield of the reaction ended up to be 77%.arrow_forwardFor the reaction of sodium sulfide with iron(III) chloride below (as an unbalanced reaction - that's how I will almost always give you reactions. ALWAYS check to see if a reaction is balanced before you use it. You balanced it in the last question; make sure you have that right before you start calculating!), you have 319.5 kg of sodium sulfide and 502.3 kg of iron(III) chloride available. What mass (in kilograms) of sodium sulfide do you NEED if you're going to reduce all of the iron(III) chloride you have? Na2S(s) + FeCl3(s) → Fe2S3(s) + NaCl(s)arrow_forward
- A reaction for the reduction of copper is shown below (as an unbalanced reaction - that's how I will almost always give you reactions. ALWAYS check to see if a reaction is balanced before you use it. You balanced it in the last question; make sure you have that right before you start calculating!), you are given 3.000 tonnes (3.000x103 kg) of copper oxide. You check and find that you have 404.9 kg of ammonia gas available to you. What mass (in kilograms) of copper(II) oxide can react with this much ammonia? CuO(s) + NH3(g) → Cu(s) + H2O(g) + N2(g)arrow_forwardHello can you please help me answer thisarrow_forwardA chemist invents a new combustion analysis instrument that uses less O2(g) which saves money on each analysis. The improvement is that instead of CO2(g) being formed and trapped, CO(g) is formed and trapped. H2O (g) is also formed and trapped when substances that contain hydrogen are analyzed.(a) The chemist places 2.184 g of an unknown compound of carbon and hydrogen in the new analyzer. 4.627 g of CO (g) and 1.795 g of H2O (g) are produced. Find the empirical formula for the compound.(Molar masses (g/mol): CO = 28.0104, H2O = 18.015) (b) Despite the best efforts of the chemist, the molar mass of the compound cannot be determined (using this method). Clearly explain why this prevents the determination of the molecular formulaarrow_forward
- Don't upload any image answer tarrow_forwardCan someone help me out with the conversion aspect of this question? Im getting the answer right but the conversion wrongarrow_forward6. Consider the following chemical reaction: 2 Cr(OH)3(aq) >Cr₂O3(s) + 3H₂0(1) 12.00 moles of chromium (III) hydroxide is decomposed. Calculate moles of the water produced. O 18.00 moles O 1.500 moles O 4.00 moles O 12.00 molesarrow_forward
- For the reaction C + 2H₂ → CH4, how many moles of hydrogen are needed to make 170.6 grams of methane, CH4 ? Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0 Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.: Element Molar Mass Hydrogen 1 Carbon 12arrow_forwardA student synthesizes alum starting with 4.924 grams of scrap aluminum. When the reaction is complete, 71.54 grams of alum are collected. What is the percent yield of this reaction? I know percent yield is actual / theoretical multiplied by 100% but I'm not sure how to identify which is theoretical or actual. Thank you for helping me!arrow_forwardPlease do not round off intermediate calculations. Thank you.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY