
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
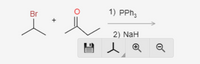
Transcribed Image Text:Br
1) PPh;
2) NaH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the balanced chemical equation for conversion of Al(s) to KAl(SO4)2·12H2O(s) in aqueous solution. Equation 1 - 2Al(s) + 2K+(aq) + 2OH-(aq) + 10H2O(l) --> 2K+(aq) + 2[Al(H2O)2(OH)4)-(aq) + 3H2(g) Equation 2 - 2K+ (aq) + 2[Al(H2O)2(OH)4]-(aq) + 2H+(aq) + SO42-(aq) --> 2K+(aq) + SO42-(aq) + 2Al(H2O)3(OH)3(s) Equation 3 - 2Al(H2O)3(OH)3(s) + 6H+(aq) + 3SO42-(aq) --> 2Al3+(aq) + 3SO42-(aq) + 12H2O(l) Equation 4 - K+(aq) + Al3+(aq) + 2SO42-(aq) --> KAl(SO4)2∙12H2O(s)arrow_forwardWrite a balanced chemical equation, including states of matter, for the combustion of gaseous benzene, C6H6.arrow_forward2. What is the theoretical yield of Compound Y if you have 8.55 g of Reactant X reacted with an excess of Cr2O72? Balance the chemical equation. OH Compound X (a) OH + Cr₂O72- OH ° Compound Y OH + Cr³+ Fill in the squares in the following chemical equations: NaOH/H₂O EtOHarrow_forward
- cigarette lighters burn butane, C4H10. write a balanced equation, assuming complete combustion, that is, plenty of oxygen.arrow_forwardStructure and Bonding Cyanamide, NH2CN, has a melting point of 45 °C. (a) Draw a dot-and-cross diagram to illustrate the electron arrangement in NH2CN. (b) Name the predominant intermolecular interaction that could account for the melting point of cyanamide, and illustrate your answer with a diagram. (a) The melting point of NaCl was found to be 801 °C. Suggest, with reasons, whether the melting point of MgO will be higher or lower than that of NaCl. 3 (Refer to Section 1.2.2, pg 4 of Topic 3 Lecture Notes to revise how to compare ionic bond strength) (b) The boiling points of ammonia, carbon monoxide and carbon dioxide are given in the table below: gas formula Mr b.p. /°C ammonia NH3 17 -33 carbon monoxide CO 28 -192 carbon dioxide CO2 44 -78 With reference to their structure and bonding, account for their boiling points. (Refer to Section 5, pg 22 of Topic 3 Lecture Notes to revise how to identify the type of IMF)arrow_forwardDraw the Lewis structure of the predominant species of H₂CO3 at a pH of 7.5.arrow_forward
- (a) Write the chemical equation for the formation of 1 mole of ethanol from its elements. Include the states of matter.arrow_forward(a) (b) (c) Br Br Nal acetone NaCN NaSCH 3arrow_forwardBecause carbon and silicon are both elements in group 14 on the periodic table, we expect them to react with other elements in similar ways. To some extent, they do, but in some cases, carbon and silicon compounds that seem to have analogous structures have very different chemical characteristics. For example, carbon tetrachloride, CCl4, is very stable in the presence of water, but silicon tetrachloride, SiCl4, reacts quickly with water. The unbalanced equation for this reaction is SiCl4 + H2O → Si(OH)4 + HCl Balance this equation. Write a conversion factor that could be used to convert between moles of SiCl4 and moles of H2O. How many moles of SiCl4 react with 24 moles of water? Write a conversion factor that could be used to convert between moles of Si(OH)4 and moles of water. How many moles of Si(OH)4 form when 4.01 moles of H2O react with an excess of SiCl4?arrow_forward
- Which of the following statements about the reaction H2(g)+O2(g)→H2O(l)H2(g)+O2(g)→H2O(l) is true? (i) This is an example of an acid–base reaction. (ii) O2O2 is oxidized in this reaction. (iii) H2H2 is reduced in this reaction. Which of the following statements about the reaction is true? (i) This is an example of an acid–base reaction. (ii) is oxidized in this reaction. (iii) is reduced in this reaction. iii only i only ii and iii ii only None of them are true.arrow_forwardA 500 megawatt electrical power plant typically burned 1,430,000 tonne of coal in a year. (a) Assuming that the coal was 80% carbon and 3% sulfur and that combustion was complete, calculate the number of tonne of carbon dioxide and sulfur dioxide produced by the plant during the year. (b) If 50% of the SO2 could be removed by reac- tion with powdered CaO to form CaSO3, how many tonne of CaSO3 would be produced?arrow_forwardConsider the series of reactions to synthesize the alum (KAl(SO4 )2 · xH2O(s)) from the introduction. (a) Assuming an excess of the other reagents, from one mole of aluminum Al (s), how many moles of alum will be produced? (b) Assuming an excess of the other reagents, from one mole of potassium hydroxide KOH, how many moles of alum will be produced? (c) Assuming an excess of the other reagents, from one mole of sulfuric acid H2SO4 , how many moles of alum will be produced? (d) If you start the synthesis with 1.00 g of Al, 40.0 mL of 1.50 M KOH, and 20.0 mL of 9.00 M H2SO4 , which of the three will be the limiting reagent? (e) Assuming that the product is anhydrous (that there are no waters of hydration), calculate the theoretical yield of alum, in grams, based on the amounts of reagents in part (d). 3. Consider the nickel salt: (NH4 )2Ni(SO4 )2 ·y H2O (Ammonium Nickel Sulfate Hydrate), where y is the number of coordinated waters. (a) Assuming that the product is anhydrous (y = 0),…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning