
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
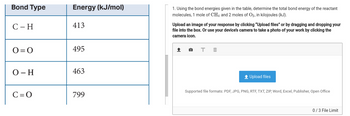
Transcribed Image Text:Bond Type
C - H
0=0
O-H
C=O
Energy (kJ/mol)
413
495
463
799
1. Using the bond energies given in the table, determine the total bond energy of the reactant
molecules, 1 mole of CH4 and 2 moles of O2, in kilojoules (kJ).
Upload an image of your response by clicking "Upload files" or by dragging and dropping your
file into the box. Or use your device's camera to take a photo of your work by clicking the
camera icon.
↑
T m
+ Upload files
Supported file formats: PDF, JPG, PNG, RTF, TXT, ZIP, Word, Excel, Publisher, Open Office
0/3 File Limit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 7. Use the bond energies provided to estimate ΔH°rxn for the reaction below. CH3OH(l) + 2 O2(g) → CO2(g) + 2 H2O(g) ΔH°rxn = ? Bond Bond Energy (kJ/mol) C-H 410 C-O 359 C=O 799 O=O 498 O-H 454arrow_forwardCHAPTER 5 - PRINCIPLES OF CHEMICAL REACTIVITY: EN Previous Page 3 of 5 endothermic ✓ exothermic When 1 mole of I2 (g) reacts with Cl₂ (g) to form ICI(g) according to the following equation, 26.8 kJ of energy are evolved. 1₂ (9) + Cl₂ (9) → 21C1(g) What is the value of q? kJ NOV 11 Next → othermic or exothermic? #tv ♫ P 100 Use the References to access DO Cengage Learning | C MacBarrow_forwardPlease answer question 2arrow_forward
- Use average bond enthalpies (linked above) to calculate the enthalpy change for the following gas-phase reaction. C2H4(g) + Br2(g) CH,BRCH,Br(g) To analyze the reaction, first draw Lewis structures for all reactant and product molecules. • Include all valence lone pairs in your answer. • Draw the reaction using separate sketchers for each species. One molecule per sketcher, please. Separate multiple reactants and/or products using the + sign from the drop-down arrow. Separate reactants from products using the If you have to draw H,, draw H-H. • Be sure that your structural equation is balanced. symbol from the drop-down menu.arrow_forwardTry Again Row 3: Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. A student runs two experiments with a constant-volume "bomb" calorimeter containing 1100. g of water (see sketch at right). First, a 7.000 g tablet of benzoic acid (CH₂CO₂H) is put into the "bomb" and burned completely in an excess of oxygen. (Benzoic acid is known to have a heat of combustion of 26.454 kJ/g.) The temperature of the water is observed to rise from 15.00 °C to 53.26 °C over a time of 12.3 minutes. Next, 5.420 g of acetylene (C₂H₂) are put into the "bomb" and similarly completely burned in an excess of oxygen. This time the temperature of the water rises from 15.00 °C to 74.42 °C. Use this information, and any other information you need from the ALEKS Data resource, to answer the questions below about this reaction: Is this reaction exothermic, endothermic, or neither? If you said the reaction was exothermic or…arrow_forwardCould someone please help! No plagiarism Please! 3. Draw an or find an image of an endothermic reaction or an exothermic reaction. Explain what makes it the type of reaction you chose to illustrate. Include arrows to show what direction the overall enthalpy of reaction (energy of reaction) flows. 4. Ammonium nitrate dissolves in water via the following reaction: NH4NO3(s) → NH4+(aq) + NO3-(aq) The bond energies of the compounds in the reaction are as follows: NH4NO3 = 3040 kJ/mol NH4+ = 1564 kJ/mol NO3- = 1009 kJ/mol How much total energy does the reaction have, and how will the environment change when the reaction occurs? Please show all of your workarrow_forward
- Question 11 X Incorrect. Isooctane, C3H18, is the basis of the octane rating system for gasoline because it burns smoothly, with minimal engine knocking. Pure isooctane is assigned a value of 100. Octane ratings are assigned to gasoline mixtures based on their ability to prevent knocking relative to isooctane. Estimate the energy released during the combustion of 1.00 mol of pure isooctane. Please note the combustion is exothermic process and the released energy is positive for the surroundings. -285.8 kJarrow_forwardplease complete 1 through 5!arrow_forwardA 3.59°C increase in temperature occurs when a 0.232 g sample of benzoic acid (C7H6O2) is combusted in a bomb calorimeter. A 1.81 °C increase in temperature occurs when a 0.303 g sample of citric acid (C6H8O7) is combusted in the same bomb calorimeter. The heat of combustion of benzoic acid is -26.37 kJ/g. What is the molar heat of combustion of citric acid? Reminder: Any heats of combustion will be negative since these are exothermic reactions.arrow_forward
- CSCI has a lattice energy of -657 kJ/mol. Consider a generic salt, AB, where A²+ has the same radius as Cs*, and B has the same radius as Cl. Estimate the lattice energy of the salt AB. lattice energy of AB: kJ/molarrow_forwardCalculate the enthalpy of reaction for the combustion reaction (in kJ/mol) shown below. Enter your answer without units. ннно: •. Н-С-С—С—С-Ӧ—Н + 50=0 4 0=c=o° + 4 Н—0—Н ннн Average Bond Energy Bond (kJ/mol) H-H 436 H-C 414 H-O 464 C-C 347 C=C 611 C-0 360 C=O 799 0-0 142 O=0 498 If you cannot see the images above,then click herearrow_forwardWhen 3.8 g A are reacted with 8.5 g D, according to the balanced chemical equation below, 33 kJ of energy are released. Please calculate the enthalpy for this reaction when 3 moles of G are produced. 2A + 3D = 2Z + 3G given molar masses: A = 141.8 g/mole D = 63.996 g/ mole Z = 4.032 g/ mole G = 56.028 g/ molearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY