
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Blackboard Learn
←
M Gmail
<
X
C A app.101edu.co
STARTING AMOUNT
Aktiv Chemistry
YouTube Maps Welcome to MyTCC
X
S
3
X b Search results for 'Det X
E
O:
4
403- Forbidden: Ac...
ADD FACTOR
* ( )
6.022 x 102
0.0288
R
g AICI,
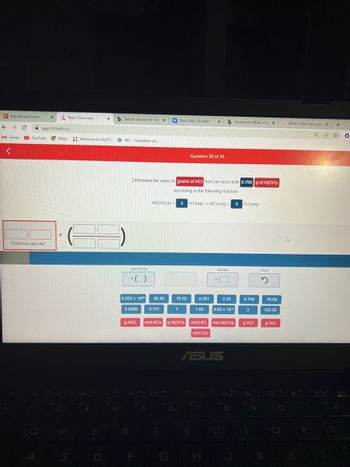
ermine the mass in grams of HCI that can react with 0.750 g of Al(OH),
according to the following reaction
Al(OH) (s) +
5
36.46
0.117
Your Sets | Quizlet
1
18.02
Question 38 of 50
3 HCl(aq) → AlCl(aq) + 3 H.O(aq)
F7
0.351
1.05
Y
X
mol H₂O
mol AICL g Al(OH), mol HCI mol Al(OH),
b Answered: What is the X
H
ASUS
FB
ANSWER
2.25
9.60 x 10
8
FO
0.750
3
g HCI
RESET
2
78.00
133.33
g H₂O
G What is the mass perc X
1.4
V12
P
+
1
Expert Solution
arrow_forward
Step 1
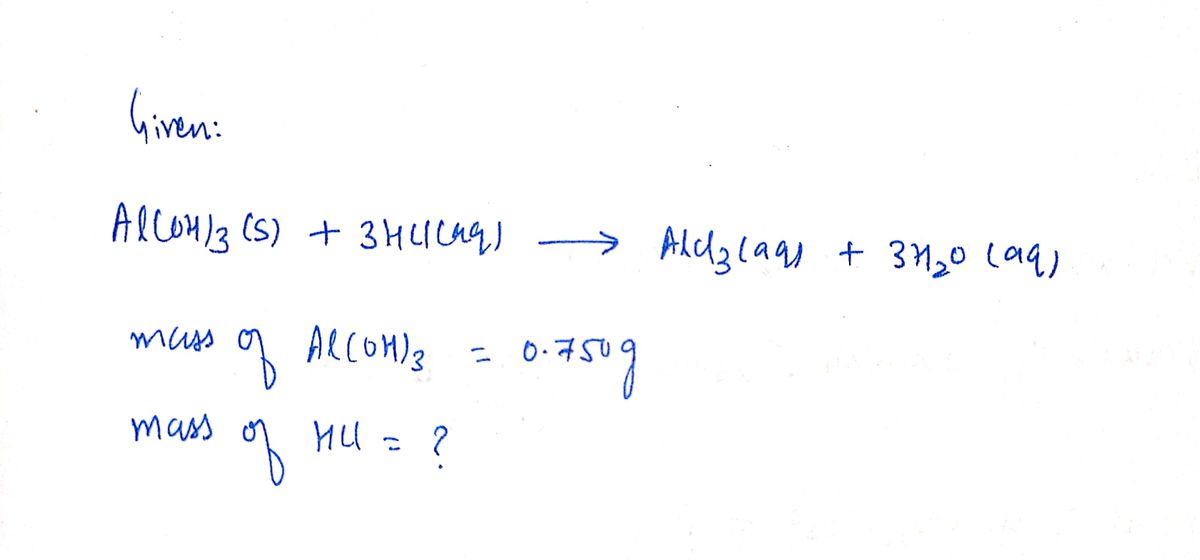
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Window Help ered: eq req Ereg Important values if needed... | b... M M 2req 2req 2req =2req M Visited MAY 11 S prod01-cnow-owl.cengagenow.com Submit Answer 85 According to the following reaction, how many moles of carbon dioxide will be formed upon the complete reaction of 32.0 grams of carbon monoxide with excess oxygen gas? 2CO(g) + O2(g) → 2CO2(g) mol carbon dioxide Retry Entire Group A 6 [Review Topics] [References] Use the References to access important values if needed for this question. Module 2 | OWLv2 MacBook Pro & 87 9 more group attempts remaining * CO € 8 A S OWLV2 | Online teaching and learning resource from C... N Previous Email Instructor Next Thu May 11 10:3 zoom □ + 0 Save and Exit + 11 bl 2 201 ngsarrow_forward12 20 A chemist adds 0.85 L of a 0.0632M barium chloride (BaCl,) solution to a reaction flask. Calculate the mass in grams of barium chloride the chemist has added to the flask. Be sure your answer has the correct number of significant digits. Submit Assignment Continue Terms of Use Prvecy Acce 02021 McGrow-H Education All Rights Resorved 99 Type here to search hparrow_forwardcollege.com/course.html?courseld=15539865&Heplb=67br| Sugar O Shop | Origami Owl Wholesale Glass... 5 The Corinthian Ho... Personalized Invi... TUII Z TJ TUT CTLIVT Course Homearrow_forward
- Calculate the mass percent of hydrogen in C6H6 to 2 significant digits. [put only the 2 digit number, with a decimal pointarrow_forwards Percent 3 Submitting an external tool ers or 7 of for n d n ▼ ▼ Q Search Part A L Submit Volume = Value HA Part B Potassium cyanide is a toxic substance, and the median lethal dose depends on the mass of the person or animal that ingests it. The median lethal dose of KCN for a person weighing 115 lb (52.2 kg) is 6.01x10-3 mol. What volume of a 0.0680 M KCN solution contains 6.01x10-3 mol of KCN? Express the volume to three significant figures and include the appropriate units. ▶View Available Hint(s) mol Previous Answers PRE Units hp www ? 4 X Incorrect; Try Again; 5 attempts remaining The value you reported corresponds to the volume of solution that contains 0.0680 mol of KCN. You need to find the volume of solution that contains 6.01×10-3 mol of KCN To find the volume of solution (L) containing the specified number of moles of solute, multiply the given moles by the concentration so that the units of mole cancel. p (3 EA ? Help 4 of 10 L Review | Constants| Periodic Tablearrow_forwardmistry app.101edu.co YouTube Maps NG AMOUNT * @ 7 2 X W S F2 X Jill # Welcome to MyTCC Welcome to MyTCC 3 F3 E D C 20: $ 4 R F 30 % 35.6 V Aluminium has a density of 2.70 g/cm² How many moles of aluminum are in a 13.2 cm block of the metal substance? 4 ADD FACTOR X x() F5 5 molecules Al T Content G 1.32 F6 A 6 B 2.70 0.489 g Al/mol F7 Y & H Question 1 of 50 0.100 g/cm² W 7 26.98 N ASUS X F8 U J g Al Lo b Home | bartleby ANSWER 8 962 13.2 F9 | M mol Al 1 1 K F10 9 < I RESET 5 6.022 x 10 O cm 1 F11 O L X + 10 P : F12 - I Prt Sc 1 ? + [ Q 12 ☆ = 12 Insert 1 Delete Backspace J Submit Home Enter PgUp Shift Pearrow_forward
- Remaining Time: 1 hour, 01 minute, 47 seconds. * Question Completion Status: QUESTION 2 The number of water molecules in 2.3 mg of water is O 6.02 x 1023 O 77.0 x 1023 O 7.7 x 1023 O 7.7 x 1019 none of the above QUESTION 3 A ctudent makes a colution bu discolvina 254a ofNDOH int0 450 gof water Wh. Click Save and Submit to save and submit. Click Save All Answers to save all answer ere to search DELLarrow_forwardm/course.html?courseld=16481757&OpenVellumHMAC=feeaec83fa5cc7b2f32454414caf19a1#10001 Part C In the gaseous state, chlorine exists as a diatomic molecule Cl2 (Molar mass = 70.9 g/mol). Calculate the number of moles of chlorine present in 140 g of chlorine gas. Express the quantity in moles to three significant figures. • View Available Hint(s) ? mol Moles of chlorine gas = Submit Previous Answers X Incorrect; Try Again Multi-step problems A problem that asked you to convert molecules to grams could require two steps 1. convert the molecules to moles P Pearson 2convert to moles to areme Copyright O 2021 Pearson Education Inc. All rights reserved. I Terms of Use | Privacy Policy I Permissions | Contact Us |arrow_forward-CHEM A054 520 2 X Question 10 - Chapter 9 part 2 X CH6_Chem103 - Kenai Peninsu x + ezto.mheducation.com/ext/map/index.html?_con=con&external_browser=0&launch Chapter 9 part 2 Homework i 10 1 points 8 01:46:48 eBook Hint Print References Mc Graw Hill 7 2 Determine the mass of Na2SO3 necessary to prepare 500.0 mL of a solution that is 0.168 M Na2SO3. g Na2SO3 3 80 00 F3 $ 4 000 000 F4 dº LO % 5 8 DII F8 ( 9 2. F9 (arrow_forward
- The answer that was given i already had the answer so I'm not sure why u guy's did the same one?...can u do problem #2. I don't need problem 1. Please give me credit for the one you answer wrong. Thank youarrow_forwardPlease help me answer thisarrow_forwardA Login My AP Login - Coll. LanguageTool -Onl.. Co Biography of Albert. Crillegnettouth Pre-AP Unit 3 Learning Checkpolnt 2 1 (3 10 11 Question 9 D CH, (g) + 2 O2 (g) CO, (g) + 2 H20 (g) When CH, (g) is burned in O,(g), the reaction represented by the equation occurs. If 32 g of CH, is burned completely, how many moles of CO, are produced? Enter the number of moles to the nearest whole number. molarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY