
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Indicate the name if the
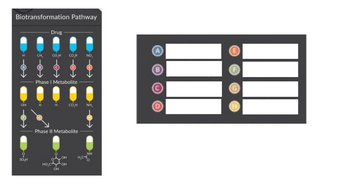
Transcribed Image Text:Biotransformation Pathway
Drug
OH
9
SO,H
CH,
CO,H CO,R NO₂
B
Phase I Metabolite
CỎ
ANH,
Phase II Metabolite
NH
OH
H,C
HO,C OH
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Consider the following biochemical reactions: H₂N-CH-C-NH-CH-C- CH3 _________ reaction A B с HO-CH2 H HO -OH H OH H H OH OH + H₂O ATP ADP enzyme 0 0 0 NADH enzyme enzyme type of enzyme needed to catalyze this reaction HỎ 2 H₂N- CH₂ NAD La pl OH -O, H он н ¸ÑLÒ¯ CH₂ H OH In the table below, write the type of enzyme that catalyzes each reaction. Note: be sure to use only the 6 standard enzyme types. OH A B сarrow_forward9. To what main enzyme class do the enzymes that catalyze the following reaction belong? (oxidoreductases, transferases, hydrolases, lyases, isomerases, ligases) HO H H₂N-C-CN-C-coo + H₂0- II HR₂ 1 R₁ This bond is cleaved H H₂N-C-Coo R₂ H₂N H 1 R₁ -COOⒸarrow_forwardAn enzymatic assay was performed using the enzyme phophofructokinase-1, which catalyses the committed step of the glycolysis pathway. What is the final concentration of the fructose 6-phosphate substrate in a test tube containing the following: 250 µL of 150pM fructose 6-phophate, 100 µL 20 mg.ml-1 phosphofructoskinase-1 and 150 µL 100mM buffer pH 7.5arrow_forward
- Oxaloacetate is an inhibitor of succinate dehydrogenase because it is structurally very similar to succinate as shown below. Also, its binding to the enzyme does not involve any covalent bond formation. What type of inhibitor is oxaloacetate? COO | CH₂ 1 CH₂ 1 COO Succinate COO | CH₂ C=O COO Oxaloacetate O a noncompetitive inhibitor both a noncompetitive and an irreversible inhibitor O an irreversible inhibitor O a competitive inhibitorarrow_forwardViagra inhibit PDE5?what does it means?what will happen if Viagra inhibit PDE5?arrow_forwardConsider the following biochemical reactions: HO H₂N A B с NH₂ reaction NH₂ ATP ADP enzyme + 2 H₂O enzyme 0 0 FAD FADH₂ ܘܐ ܀ enzyme type of enzyme needed to catalyze this reaction HO dy dy OH In the table below, write the type of enzyme that catalyzes each reaction. Note: be sure to use only the 6 standard enzyme types. NH₂ + 2 NH3 X Ś A Barrow_forward
- Absorbed from the intestines into the blood, nitrites (NO2-) interact with the hemoglobin of the blood and block its respiratory function, turning part of the hemoglobin (HbFe2+) into methemoglobin (HbFe3+), unable to transfer oxygen from the lungs to tissues. With the formation of a large amount of methemoglobin, oxygen starvation of tissues occurs, which can cause damage to the central nervous system. 10 to 20 % - of methemoglobin (HbFe3+) - asymptomatic cyanosis, 20 to 50% of methemoglobin (HbFe3+) -hypoxia develop, > 50% of methemoglobin (HbFe3+) - the person will die. The body weight of the average person is 60 kg. Blood mass averages 8% of a person’s body weight; blood density ρ = 1,050 g/cm3, the hemoglobin (Hb) content in it is 14 g per 100 ml. molecular weight of hemoglobin 65-68 kg/mol (use 68 kg/mol for calculation). Assume that 1 mole of hemoglobin reacts with one mole of nitrite ion to form 1 NO molecule: HbFe2+ + NO2- → HbFe3+ + NO will a 290 mg dose of sodium nitrite…arrow_forward8 The optimal temperature for the action of lactate dehydrogenase is 36°C. It is irreversibly inactivated at 85°C, but a yeast containing this enzyme can survive for months at —10°C. Explain how this can happen.arrow_forwardWrite and discuss the mechanism of the reaction that happens at the active site of the enzyme cathecol oxidase.arrow_forward
- Mechanism of drug action is explored by: * pharmacoeconomics pharmacodynamics O pharmacogenetics O pharmacokineticsarrow_forward6. What class do the enzymes that catalyze the following reaction belong? (oxidoreductases, transferases, hydrolases, lyases, isomerases, ligases) H H H. C=C H-----H Hi H. H H ↑arrow_forwardAcetolactate synthase transfers the acyl group of pyruvate to alpha-ketobutyrate. This is the first step in the biosynthesis of the amino acid isoleucine. Propose a mechanism for this reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning