
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Transcribed Image Text:Saved
3 attempts left
Check my work
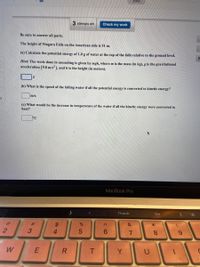
Be sure to answer all parts.
The height of Niagara Falls on the American side is 51 m.
(a) Calculate the potential energy of 1.0 g of water at the top of the falls relative to the ground level.
Hint: The work done in ascending is given by mgh, where m is the mass (in kg), g is the gravitational
acceleration (9.8 m/s² ), and h is the height (in meters).
(b) What is the speed of the falling water if all the potential energy is converted to kinetic energy?
m/s
(c) What would be the increase in temperature of the water if all the kinetic energy were converted to
heat?
°C
MacBook Pro
Thank
We
23
$4
&
3
4.
8.
W
E
Y
U
w/
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Be sure to answer all parts. Agas expands from 265 mL to 948 mL at a constant temperature. Calculate the work done (in joules) by the gas if it expands (a) against a vacuum. WH (b) against a constant pressure of 4.00 atm.arrow_forwardThe heat capacity of a bomb calorimeter was determined by burning 6.85 g of methane (energy of combustion = -803 kJ/mol CH4) in the bomb. The temperature changed by 11.1°C. (a) What is the heat capacity of the bomb? kJ/°C (b) A 14.7-g sample of ethanol (C₂H5OH) produced a temperature increase of 12.8°C in the same calorimeter. What is the energy of combustion of ethanol (in kJ/mol)? kJ/molarrow_forwardCalculate Unit costarrow_forward
- How much work (energy) is required to lift a 173 g object by a height of 71.0 feet? The earth's gravitational constant is g = 9.80 m/s2.arrow_forwardA 1.70-mol sample of hydrogen gas is heated at constant pressure from 306 K to 414 K. (a) Calculate the energy transferred to the gas by heat. (Enter your answer to at least two decimal places.) kJ(b) Calculate the increase in its internal energy. (Enter your answer to at least two decimal places.) kJ(c) Calculate the work done on the gas. kJarrow_forwardThe distance between Sherbrooke and Montreal is 137km. However, a car driving between the two points travels 156km. Of these distances, which one is analogous to a state function?arrow_forward
- and 2 Enthalpy #1.30 enoindice pled TT 2010 2D49.bs 22) When 1 mole of Fe2O3(s) reacts with H₂(g) to form Fe(s) and H2O(g) according to the following equation, 98.8 kJ of energy are absorbed. (6 Fe2O3(s) + 3 H2(g) → 2 Fe(s) + 3 H₂O(g) (pp) (20/d4 (pm) $40 D Reaclanis 13:48 SH Products endothermic, A endothermic, B (2) exothermic, A exothermic, B nad W (21 Enthalpy MOMS. Reactants Products (A) (B) Is the reaction endothermic or exothermic, and which of the enthalpy diagrams above represents grow olor and to noin (01 this reaction? SH GH TH A HOMH X HILD 08.10 Page 6 of 8arrow_forwardA gas expands from 287 mL to 959 mL at a constant temperature. Calculate the work done (in joules) by the gas if it expands (a) against a vacuum. W = J (b) against a constant pressure of 4.00 atm. W = Jarrow_forwardA bomb calorimeter, or constant volume calorimeter, is a device often used to determine the heat of combustion of fuels and the energy available from foods. Since the "bomb" itself can absorb energy, a separate experiment is needed to determine the heat capacity of the calorimeter. This is known as calibrating the calorimeter. In the laboratory a student burns a 0.508-g sample of benzil (C14H1002) in a bomb calorimeter containing 1060. g water. The temperature increases from 24.70 °C to 27.80 °C. The specific heat capacity of water is 4.184 J gl°C1. The combustion enthalpy is -6784 k]/mol benzil. C14H1002(s) + 31/2 02(g) →14 CO2(g) + 5 H20(I) A,H° = -6784 kJ/mol %3D Calculate the heat capacity of the calorimeter. heat capacity of calorimeter = J/Carrow_forward
- 5.00 × 10−2 mol KOH and an excess of hydrochloric acid are combined in 100.0 mL of water initially at 25.0 °C, leading to a final measured temperature of 31.1 °C. (a) What is the heat flow for the water in the calorimeter, qH2O, in Joules? (b) Assuming the calorimeter constant Ccal = 50.0 J °C , what is the heat flow for the calorimeter, qcal, in Joules? (c) What is the amount of heat given off by the reaction, qrxn, in Joules? (d) What is the molar enthalpy of the neutralization reaction (shown below) as measured in the calorimeter, ∆H◦ rxn in kilojoules per mole? H3O + (aq) + OH– (aq) → 2H2O (l)arrow_forwardCalculate the molar heat capacity (cm) for Iron (Fe). cFe = 0.451 J/(g•oC) Express your answer 3 significant figures, and without the units.arrow_forwardCalculate AE and determine whether the process is endothermic or exothermic for the following cases: (1) q = 0.123 kJ and w = - 230 J (2) A system releases 66.1 kJ of heat to its surroundings while the surroundings to 44.0 kJ of work on the system.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY