
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
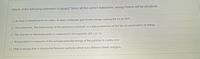
Transcribed Image Text:Which of the following statement is correct? Select all the correct statements, wrong choices will be penalized.
O As heat is transferred to ice cubes, its water molecules gain kinetic energy, causing the ice to melt.
OThe statement, "the total energy of the universe is constant", is a logical extension of the law of conservation of energy.
O The first law of thermodynamics is expressed in the equation, AH = q + w.
O Temperature is a measure of the average potential energy of the particles in a substance
O Heat is energy that is transferred between particles which have different kinetic energies.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- II. Rapid walking through a distance of 2.2 km for 30 minutes per day requires 41 J while swimming for the Same penod of time through the same distance requires 226 J. Will these forms of exercise allow you to lose weight equivalent to ig of glucose (MW = 180 g/mol) in 1 week? The standard enthalpy of combustion of glucose is -2806 kJ.arrow_forwardFor a process, 153 J of heat was released by the system and 267 J of work was done on the system. Calculate the change in internal energy, A E, for this process. i Jarrow_forwardCHAPTER 5 - PRINCIPLES OF CHEMICAL REACTIVITY: EN Previous Page 3 of 5 endothermic ✓ exothermic When 1 mole of I2 (g) reacts with Cl₂ (g) to form ICI(g) according to the following equation, 26.8 kJ of energy are evolved. 1₂ (9) + Cl₂ (9) → 21C1(g) What is the value of q? kJ NOV 11 Next → othermic or exothermic? #tv ♫ P 100 Use the References to access DO Cengage Learning | C MacBarrow_forward
- When methanol, CH, OH, is burned in the presence of oxygen gas, O,, a large amount of heat energy is released. For this reason, it is often used as a fuel in high performance racing cars. The combustion of methanol has the balanced, thermochemical equation CH, OH(g) + 0,(g) → CO,(g) + 2 H,O(1) ΔΗ --764 kJ How much methanol, in grams, must be burned to produce 837 kJ of heat?arrow_forwardCalculate the energy required to heat 1.80kg of ammonia from 21.5°C to 41.0°C . Assume the specific heat capacity of ammonia under these conditions is ·4.70J·g−1K−1 Round your answer to 3 significant digits.arrow_forwardA chemist carefully measures the amount of heat needed to raise the temperature of a 0.88 kg sample of C,H0, from 35.8 °C to 51.4 °C. The experiment shows that 2.34 × 10* J of heat are needed. What can the chemist report for the molar heat capacity of C,H00,? Round your answer to 2 significant digits. |J-mol 1 - 1 •K х10arrow_forward
- Which statement is incorrect? Energy is the capacity to do work or to transfer heat. Kinetic energy is the energy of motion O Aprocess that absorbs energy from its surroundings is called exothermic. The Law of Conservation of Energy is another statement of the First Law of Thermodynamicsarrow_forwardA bomb calorimeter, or constant volume calorimeter, is a device often used to determine the heat of combustion of fuels and the energy available from foods. Since the "bomb" itself can absorb energy, a separate experiment is needed to determine the heat capacity of the calorimeter. This is known as calibrating the calorimeter. In the laboratory a student burns a 0.461-g sample of 2-naphthylacetic acid (C12H1002) in a bomb calorimeter containing 1150. g water. The temperature increases from 24.70 °C to 27.20 °C. The specific heat capacity of water is 4.184 J g1 oC-1. The combustion enthalpy is -5779 kJ/mol 2-naphthylacetic acid. C12H1002(s) + 27/2 02(g) –→12 CO2(g) + 5 H20(1) A,H° -5779 kJ/mol Calculate the heat capacity of the calorimeter. heat capacity of calorimeter= orc J/Carrow_forwardA system does 501 kJ of work and loses 216 kJ of heat to the surroundings. What is the change in internal energy, AE, of the system? Note that internal energy is symbolized as AU in some sources. AE = kJarrow_forward
- What is the mas of natural gas (CH4) must you burn to emit 276 kJ of heat?arrow_forwardPart A A silver block, initially at 55.8 °C, is submerged into 100.0 g of water at 24.1°C in an insulated container. The final temperature of the mixture upon reaching thermal equilibrium is 26.3 °C. The specific heat capacities for water and silver are = 4.18 J/(g ·°C) and 0.235 J/(g·°C). What is the mass of the silver block? Express your answer to two significant figures and include the appropriate units. Cs, water • View Available Hint(s) Cs, silver = HA ? Value Units m =arrow_forwardThe work done to compress a gas is 87 J. As a result, 56 J of heat is given off to the surroundings. What is the change in the internal energy (in J) of the gas? Note: Report your answer in whole number, without decimal place (Example: 20 not 20.0).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY