
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
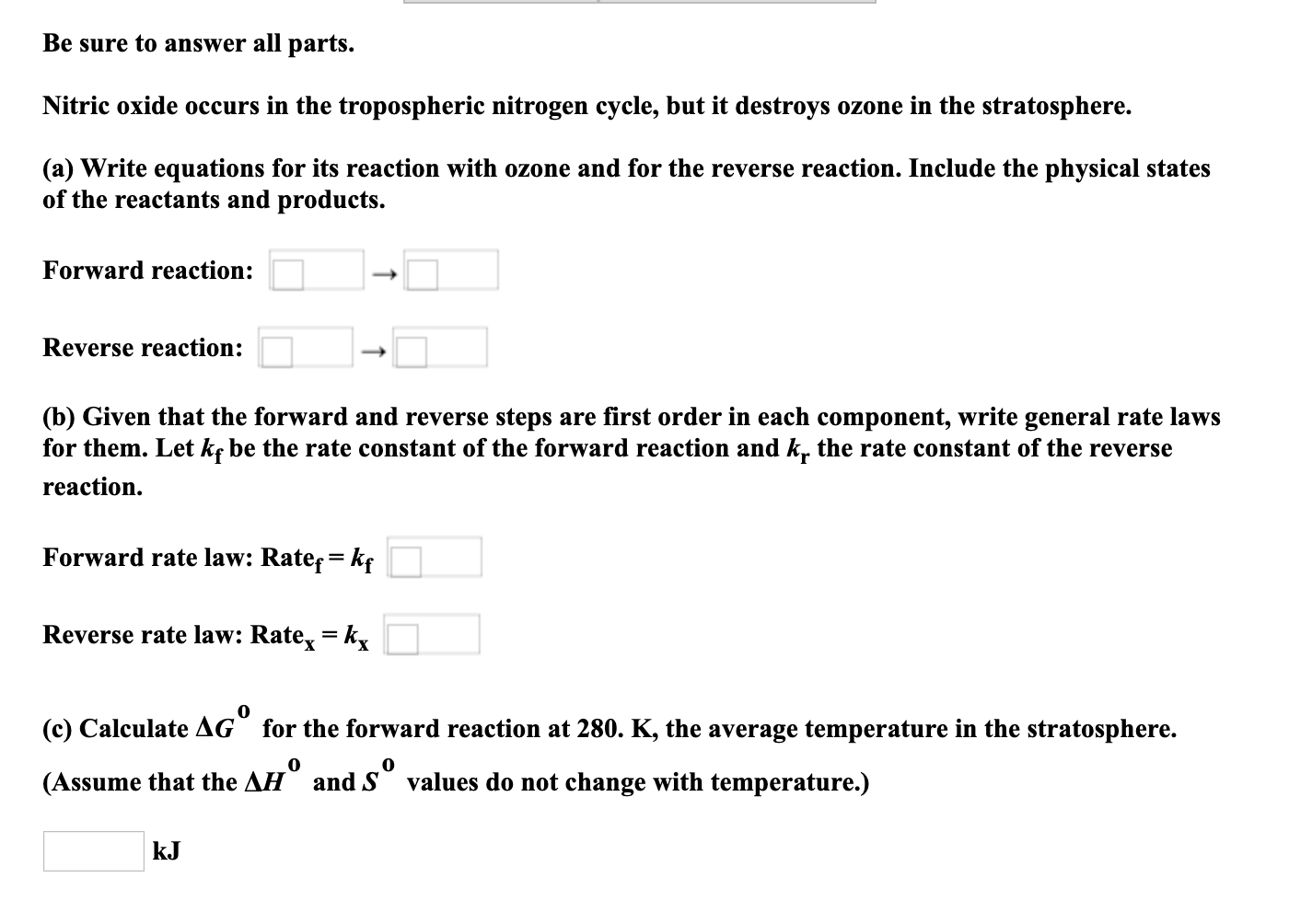
Transcribed Image Text:Be sure to answer all parts.
Nitric oxide occurs in the tropospheric nitrogen cycle, but it destroys ozone in the stratosphere.
(a) Write equations for its reaction with ozone and for the reverse reaction. Include the physical states
of the reactants and products.
Forward reaction:
Reverse reaction:
(b) Given that the forward and reverse steps are first order in each component, write general rate laws
for them. Let kç be the rate constant of the forward reaction and k. the rate constant of the reverse
reaction.
Forward rate law: Rate= kf
Reverse rate law: Rate, = k,
(c) Calculate AG for the forward reaction at 280. K, the average temperature in the stratosphere.
(Assume that the AH and S values do not change with temperature.)
kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- The OH radical reacts with itself in a disproportionation type reaction according to the reaction: OH + OH → H2O + O This rate constant at room temperature is 2.69 x 102 M-1 s-1. If the initial concentration of OH is 2.60 x 10-5 M, what is the first half life for the reaction (in seconds)? (the answer should be entered with 3 significant figures; do not enter units; give answer in scientific notation--valid notation examples include 1.23e-8 and 1.23e8 and -1.23e-4 and 1.23e0)arrow_forward::(:(/(/(/(//arrow_forwardSuppose the formation of dinitrogen pentoxide proceeds by the following mechanism: step elementary reaction 1 NO₂ (g) +03 (g) → NO3 (g) + O₂(g) 2 NO3 (g) + NO₂ (g) → N₂O5 (g) Suppose also k₁ « k₂. That is, the first step is much slower than the second. Write the balanced chemical equation for the overall chemical reaction. Write the experimentally- observable rate law for the overall chemical reaction. Note: your answer should not contain the concentrations of any intermediates. rate = k rate constant k₁ k₂ ロ→ロ X Śarrow_forward
- Here is a graph of the molarity of thionyl chloride (SOC1₂) in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the table below. M 0.003 0.00211 0.002 0.001 0 y 10 15 seconds 20 25 30 X Śarrow_forward(1 Try Again Your answer is incorrect. • Row 3: Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. Here is a graph of the molarity of formic acid (HCO₂H) in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the table below. 3 0.030 0.025 0.020 0.0187 0.015 0.010- 0.005 0 y 500 1000 1500 seconds 2000 2500 3000 × Śarrow_forwardThe gas phase decomposition of dinitrogen pentoxide at 335 K 2 N205 →4 NO2+02 has the following relationship between rate and concentration, where the bracket around the formula indicates concentration and k is a constant called the rate constant. Rate = k[N2O5l %3D If the value of k is 7.38×10~$ s', what is the rate of the reaction when [N2O5] = 0.180 M? Ms-arrow_forward
- The following data were obtained for the reaction of carbon monoxide with chlorine, producing phosgene, COCl2, a highly toxic gas CO(g) + Cl2(g) → COCl2(g) From the following data, obtained at 360 K, determine: a) The order of the reaction, and the reaction rate law b) The value of the reaction rate constant, k c) The initial reaction rate for the formation of COCl2 when the concentration of CO is 0.30 M and that of Cl2 is 0.40 M The following data were obtained for the reaction of carbon monoxide with chlorine, producing phosgene, COCl2, a highly toxic gas CO(g) + Cl2(g) → COCl2(g) From the following data, obtained at 360 K, determine: a) The order of the reaction, and the reaction rate law b) The value of the reaction rate constant, k c) The initial reaction rate for the formation of COCl2 when the concentration of CO is 0.30 M and that of Cl2 is 0.40 M The following data were obtained for the reaction of carbon monoxide with chlorine, producing phosgene, COCl2, a highly toxic…arrow_forwarda)A nickel catalyst is commonly used in the hydrogenation of ethylene. If the initial concentration of ethylene is 2.75 mol·L−1 and the rate constant for the reaction is 0.0018 mol·L−1·s−1, what is the rate of reaction if it follows a zero-order reaction mechanism? Express your answer to two significant figures. b)Determine the half-life for the reaction in Part B. Express your answer to two significant figures.arrow_forwardConsider the following reaction: 2A + B + 3C. (a) Write an expression for the overall rate; (b) What are the rates at which A and B are consumed and generated if C is created at a rate of 0.015 M/min?arrow_forward
- The gas phase reaction between hydrogen and iodine is proposed to occur as follows: step 1: 12 →21 step 2: step 2 H₂ + 2 I (a) Identify the molecularity of each step in the mechanism. step 1 → 2 HI + (b) Write the equation for the net reaction. Use the smallest integer coefficients possible. If a box is not needed, leave it blank. + (c) Identify any intemediates in this mechanism. Intermediate: Enter formula. If none, leave box blank:arrow_forwardChlorine monoxide (ClO) accumulates in the stratosphere above Antarctica each winter and plays a key role in the formation of the ozone hole above the South Pole each spring. Eventually, ClO decomposes according to the equation: 2C1O (g) → Cl2 (g) + O2 (g) The kinetics of this reaction were studied in a laboratory experiment at 298K, and the data are shown in the table below. (a) Determine the order of the reaction (b) Determine the rate law expression (c) Determine the value of the rate constant, k, at 298K. Time (ms) [CiO] (M) In [ClO] cioj (M-1) [CiO] 6.67 × 107 1.39 x 108 -8- 1.50 x 10 7.19 x 10 4.74 × 10 -18.0 6- 10 -18.8 6- 20 -19.2 2.11 x 108 6- 30 3.52 х 10° -19.5 2.84 x 108 6- 40 2.81 x 10 -19.7 3.56 x 108 6- 100 1.27 x 10 -20.5 7.87 x 108 10 200 6.60 x 10- -21.1 1.52 x 10°arrow_forwardA. Consider the following proposed mechanism for the production of chlorine dixoide from chlorine and ozone. Draw a reaction coordinate diagram for this reaction. Label reactants, products, transition states, intermediates, and activation energy on your sketch. Assume the reaction is endothermic. NOTE: CI is chlorine, not Carbon lodide Overall: Cl₂ (g) + 203 (g) → 2 CIO₂ (g) + O₂ (g) Step 1: Cl₂ (g) → 2 Cl (g) fast Step 2: Cl (g) + Os (g) CIO₂ +O fast Step 3: O (g) + O3 (g) → 202 (g) fast Step 4: Cl (g) + O2 (g) → CIO2 (g) slowarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY