
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
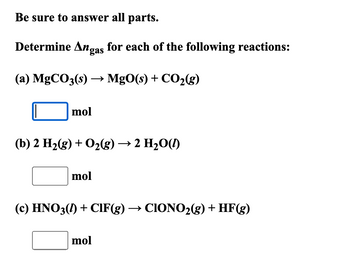
Transcribed Image Text:Be sure to answer all parts.
Determine Angas for each of the following reactions:
(a) MgCO3(s) → MgO(s) + CO2(g)
mol
(b) 2 H2(g) + O2(g) → 2 H₂O(l)
mol
(c) HNO3(1) + CIF(g) → CIONO2(g) + HF(g)
mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 21. (a) Write the balanced chemical equation for the combustion of propane (C3H3). How does the potential energy change between the reactants and products in this reaction? (b) Draw a potential energy diagram for this chemical reaction. (c) What is the relationship between these energy changes and the breakdown and/or formation of covalent bonds? Explain your ideas in at least two (2) sentences and remember to use vocabulary terms.arrow_forward(A) Write the balanced chemical equation for the formation of butane (C4H10). How does the potential energy change between the reactants and products in this reaction? (B) draw a potential energy diagram for this chemical reaction. (C) what is the relationship between energy changes and the breakdown and/or formation of covalent bonds? Explain your ideas in at least two sentences and remember to use vocabulary terms.arrow_forwardBalance the following equations. (Use the smallest whole numbers possible. Enter 1 where appropriate - do not leave any answer box blank.) (a) CAH100() + ]O2(@) → CO,(g) + H20() (b) CH4(g) + |a,(g) → CCI,(1) + HCI(g) (c) Fe(OH)2(s) + H2S(g) - Fe S3(s) + H20(g)arrow_forward
- Balance the following equations. (Use the lowest possible whole number coefficients. Include states-of- matter in your answers.) (a) Mn(s) + O2(g) → Mn2 07 (8) + (b) N₂(g) + O2(g) → N₂O(g) + (c) C10H22 (1) + O2(g) → CO₂(g) + H₂O(1) +arrow_forwardThe organic molecules shown here are derivatives of benzene in which six-membered rings are “fused” at the edges of the hexagons. (a) Determine the empirical formula of benzene and of these three compounds. (b) Suppose you are given a sample of one of the compounds. Could combustion analysis be used to determine unambiguously which of the three it is? (c) Naphthalene, the active ingredient in mothballs, is a white solid. Write a balanced equation for the combustion of naphthalene to CO2(g) and H2O(g). (d) Using the Lewis structure for naphthalene and the average bond enthalpies in Table 8.4, estimate the heat of combustion of naphthalene in kJ/mol. (e) Would you expect naphthalene, anthracene, and tetracene to have multiple resonance structures? If so, draw the additional resonance structures for naphthalene. (f) Benzene, naphthalene, and anthracene are colorless, but tetracene is orange. What does this imply…arrow_forwardWrite a balanced chemical equation for the hypothetical reaction of liquid dichloromethane, CH2CI2, with solid NaAaBb3 to form solid CH2(AaBb3)2 and another product.arrow_forward
- What mass of methane is required to produce 1.130 kg of carbon dioxide during combustion? (Assume the reaction undergoes complete combustion.) CH4(g) + O2(g) → CO2(g) + H2O(g) O 411.8 g O 113.2 g O 24.5 garrow_forwardUsing the table provided, what is the enthalpy for the reaction shown below: 6H₂(g) + 4NO(g) + CH₂(g) → CO₂(g) + 2H₂O(l) + 4NH3 (9)arrow_forwardEthanol, C₂H6 O, is most often blended with gasoline - usually as a 10 percent mix - to create a fuel called gasohol. Ethanol is a renewable resource and ethanol-blended fuels, like gasohol, appear to burn more efficiently in combustion engines. The combustion of one mole of ethanol releases 326.7 kcal of energy. The combustion of one mole of octane, C8 H18, releases 1.308 × 10³ kcal of energy. How much energy is released during the complete combustion of 460. grams of octane? kcal Assuming the same efficiency, would 460. grams of ethanol provide more, less, or the same amount of energy as 460. grams of octane? D more less the same amount Retry Entire Group 8 more group attempts remainingarrow_forward
- Don't provide handwriting solutionsarrow_forwardChoose the reaction that represents the combustion of C 6H 12O 2. C6H12O2(l) + 8 O2(g) → 6 CO2(g) + 6 H2O(g) Mg(s) + C6H12O2(l) → MgC6H12O2(aq) 6 C(s) + 6 H2(g) + O2(g) → C6H12O2(l) C6H12O2(l) → 6 C(s) + 6 H2(g) + O2(g) None of these represents the combustion of C6H12O2.arrow_forwardThe first two steps in the industrial synthesis of nitric acid produce nitrogen dioxide from ammonia: 4 NH3(g) +50₂ (g) = 4 NO(g) + 6H₂O(g) K₁ K2 2 NO(g) + O₂(g) 2 NO₂ (g) = The net reaction is: K 4 NH3(g) +70₂ (g) 4NO₂(g) + 6H₂O(g) Write an equation that gives the overall equilibrium constant K in terms of the equilibrium constants K, and K₂. If you need to include any physical constants, be sure you use their standard symbols, which you'll find in the ALEKS Calculator. K = 0 0.0 00 09 ? olo X Garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning