
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
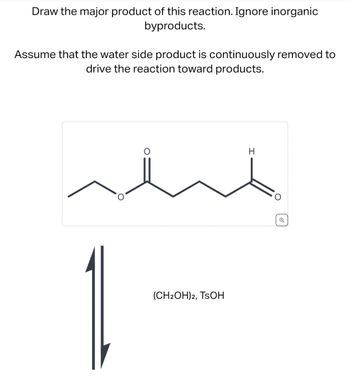
Transcribed Image Text:Draw the major product of this reaction. Ignore inorganic
byproducts.
Assume that the water side product is continuously removed to
drive the reaction toward products.
(CH2OH)2, TSOH
H
Q
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- Which compound is the dominant product in the reaction shown below? KMNO4 .COOH (A) H*/H,O (B) COOH .COOH (C) (D) HOOC HOOC Compound B Compound C Compound D Compound Aarrow_forwardPlease don't provide handwriting solutionarrow_forward10:54 ← Question 21 of 32 Draw the major product of this reaction. Ignore inorganic byproducts. Submit Assume that the water side product is continuously removed to drive the reaction toward products. (CH3)2NH, TSOH Select to Draw | I Iarrow_forward
- Please don't provide handwriting solutionarrow_forwardGive the major product of the following reaction. 1) OsO4 ? 2) NaHSO3, H2O Ph, он "ph H' Но Ph H OH Но Ph H' Ph Ph Ph Ph Ph Ph C There is no reaction under these conditions or the correct product is not listed here.arrow_forwarda) b) - Fill in the reagents above/below the arrow in the following reactions. c) CN F CO₂H OH Хонarrow_forward
- Draw the major product of this acid-base reaction. Include all lone pairs. Ignore inorganic byproducts. :0: H NaNH2 Qarrow_forwardRank the compounds in each set from most to least reactive in an EAS reaction OH I. II. COOH |||arrow_forwardWhen trichloroacetaldehyde is dissolved in water, almost all of it is converted to the hydrate. Chloral hydrate, the product of the reaction, is a sedative that can be lethal. A cocktail laced with it is known—in detective novels, at least—as a “Mickey Finn.” Explain why an aqueous solution of trichloroacetaldehyde is almost all hydrate.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning