
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
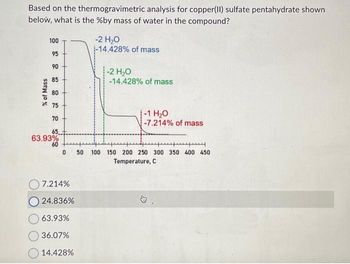
Transcribed Image Text:Based on the thermogravimetric analysis for copper(II) sulfate pentahydrate shown
below, what is the %by mass of water in the compound?
100
95
90
85
80
75
70
65
63.93%
60
% of Mass
0
7.214%
O24.836%
63.93%
36.07%
14.428%
-2 H₂O
-14.428% of mass
-2 H₂O
-14.428% of mass
-1 H₂O
-7.214% of mass
y
50 100 150 200 250 300 350 400 450
Temperature, C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Mass of evaporating dish 69.148 Mass of evaporating dish and KCl solution 77.885 Mass of KCl solution 8.737 Mass of evaporating dish and Kcl residue 71.125 Mass of Kcl residue 1.977 Find the mass of water of evaporated Percent Kcl (by mass) in solution Volume of KCl solution (density of KCl solution is 1.12 g/mL) Moles of KCl residue Molarity of solutionarrow_forwardCalculate the mass percent of a vinegar solution with a total mass of 97.02 g that contains 2.28 g of acetic acid.arrow_forwardEnter your answer in the provided box. Calculate the percent by mass of the solute in the following solution. 4.00 g of toluene in 23.0 g of benzene %arrow_forward
- Calculate the number of moles of barium chloride in 427 g of a 3.82% by mass barium chloride solution? molarrow_forwardHow many milliliters of an 8.56% solution can be prepared from 42.8g of sucrose?arrow_forwardPart A The solution concentration unit of molarity (M) is related to the moles of solute and volume of solution as follows: Moles solute Molarity (M) Liters solution Solutions are sometimes prepared from a solute that is already dissolved, and one reason for the need to do so is if the solute is not stable in air. When preparing a solution of a specific concentration from a more concentrated solution, first add the volume of solution that contains the number of moles needed, and then fill to the requisite volume with solvent. Hypochlorites, which are strong oxidizers, are examples of such compounds. Sodium hypochlorite, NaOCI, is the active ingredient in many "bleach" products. Suppose you were preparing 1.0 L of a bleaching solution in a volumetric flask, and it calls for 0.24 mol of NaOCl. If all you had available was a jug of bleach that contained 0.73 M NAOCI, what volume of bleach would you need to add to the volumetric flask before you added enough water to reach the 1.0 L line?…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY