
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Transcribed Image Text:Part A
The solution concentration unit of molarity (M) is related to the moles of solute and volume of solution as follows:
Moles solute
Molarity (M)
Liters solution
Solutions are sometimes prepared from a solute that is already dissolved, and one reason for the need to do so is if the solute is not stable in air. When preparing a solution of a specific
concentration from a more concentrated solution, first add the volume of solution that contains the number of moles needed, and then fill to the requisite volume with solvent. Hypochlorites,
which are strong oxidizers, are examples of such compounds. Sodium hypochlorite, NaOCI, is the active ingredient in many "bleach" products.
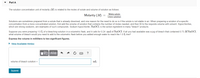
Suppose you were preparing 1.0 L of a bleaching solution in a volumetric flask, and it calls for 0.24 mol of NaOCl. If all you had available was a jug of bleach that contained 0.73 M NAOCI,
what volume of bleach would you need to add to the volumetric flask before you added enough water to reach the 1.0 L line?
Express the volume in milliliters to two significant figures.
• View Available Hint(s)
?
volume of bleach solution =
mL
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In a student experiment, the empirical formula of a copper halide was found by adding aluminum metal to an aqueous solution of the halide, displacing copper metal. The copper metal was filtered, washed with distilled water, dried, and weighed; three separate determinations were performed. The copper halide solution contained 42.62 g of copper chloride per liter. The student recorded the following experimental data. Part A Write the empirical formula of copper chloride based on the experimental data. Volume of copper chloride solution Mass of filter paper Mass of filter paper with copper (g) Trial Express your answer as a chemical formula. (mL) (g) A 49.6 0.908 1.694 > View Available Hint(s) 48.3 0.922 1.693 42.2 0.919 1.588arrow_forwardA student prepared a standard copper solution by combining 2.63 mL of stock 0.270 mol/L copper(II) sulfate solution with 6.37 mL of water. What is the molar concentration of Cu2+ in this solution? Give your answer in in decimal format. When entering units, use proper abbreviated units with proper capitalization.arrow_forwardA chemist prepares a solution of silver(I) nitrate (AgNO,) by measuring out 245. µmol of silver(I) nitrate into a 400. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in umol/L of the chemist's silver(I) nitrate solution. Be sure your answer has the correct number of significant digits. u mol x10arrow_forward
- Acetaldehyde (C₂H4O), an oxidation product of ethanol, is found in every type of wine. On average, red wine contains 30.0 mg of acetaldehyde per liter. What is the molarity of acetaldehyde in a bottle of wine (750. mL)? Do not include units or use scientific notation on your final answer (on the blank).arrow_forwardWhen answering this problem, report the answer with the appropriate number of significant figures. When entering units, use proper abbreviated units with proper capitalization. A student prepared a standard copper solution by combining 4.12 mL of stock 0.3926 mol/L copper(II) sulfate solution with 5.92 mL of water. What is the molar concentration of Cu2+ in this solution? Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: __________x10 ___power ____unitsarrow_forwardThe concentration of a stock solution of potassium dichromate is 0.50 mol/L. If you were to take 200 mL of this dilution and add 200 mL of water to get a total volume of 400 mL, what do you predict the concentration of the diluted solution would be ?arrow_forward
- A chemist prepares a solution of iron (II) chloride (FeCl₂) by measuring out 1.0 g of FeCl₂ into a 250. mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Canions in the chemist's solution. Be sure your answer is rounded to 2 significant digits. 7 mol L 0x x10 X Śarrow_forwardSuppose 20.8 g of lead(II) nitrate is dissolved in 150. mL of a 0.70 M aqueous solution of ammonium sulfate. Calculate the final molarity of nitrate anion in the solution. You can assume the volume of the solution doesn't change when the lead(II) nitrate is dissolved in it. Be sure your answer has the correct number of significant digits.arrow_forwardA chemist prepares a solution of magnesium fluoride (MgF, by measuring out 0.032 umol of magnesium fluoride into a 450. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in umol/L of the chemist's magnesium fluoride solution. Be sure your answer hås the correct number of significant digits, u mol Larrow_forward
- A chemist prepares a solution of barium acetate (Ba(CH,CO2),) by measuring out 1.3 x 10² µumol of barium acetate into a 450. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mmol/L of the chemist's barium acetate solution, Be sure your answer has the correct number of significant digits. mmolarrow_forwardYou are given solutions of HCl and NaOH and must determine their concentrations. You use 30.0 mL of NaOH to neutralize 100.0 mL of HCl, and 20.0 mL of NaOH to neutralize 50.0 mL of 0.447 M H2SO4. What is the concentration of the HCl solution? Enter your answer in units of M to three significant figures.arrow_forward4) Write a chemical equation describing the dissolution of Ba(NO3)2 in water. To give you an example of what I'm looking for, for NaBr, the equation would be NaBr(s) → Na*(aq) + Br- (aq). 5) Based on your equation from question 4, if 41.9 g of Ba(NO3)2 are dissolved in water to produce a total solution volume of 495 mL, determine the molarity of NO3- in the solution?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY