
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
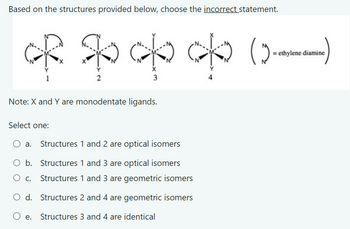
Transcribed Image Text:Based on the structures provided below, choose the incorrect statement.
ethylene diamine
(c) do op of to
2
3
Note: X and Y are monodentate ligands.
Select one:
○ a. Structures 1 and 2 are optical isomers
b. Structures 1 and 3 are optical isomers
Structures 1 and 3 are geometric isomers
О с.
○ d. Structures 2 and 4 are geometric isomers
○ e.
Structures 3 and 4 are identical
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Match the orbitals below to the higher and lower energy levels when an octahedral complex undergoes ligand field splitting. dx2-y2 Orbital 1 Orbital 1 Orbital 2 Orbital 3 Orbital 4 Orbital 5 L d₂2 Orbital 2 L dyz Orbital 3 dxz Orbital 4 [Choose] ◊ [Choose] ↑ [Choose] ◊ [Choose] [Choose] ↑ L dxy Orbital 5arrow_forwardWhich statements are true regarding ligands? Select all that apply. Ligands donate all electrons involved in bonds they form with metal cations. Ligands are electron pair acceptors in coordination complexes. Ligands act as Lewis bases in coordination complexes. Ligands form ionic bonds with transition metals. All ligands with two lone pairs are bidentate. A ligand that can donate more than one electron pair to a metal cation is called a chelate. One ligand can donate only one lone pair to a metal cation.arrow_forwardPlease answer my question!!arrow_forward
- 2. Short questions, all-or-nothing. There may be more than one answer per question. axial 3- CN NC Ni CN CN equatorial NC а. For the square base pyramidal complex [Ni(CN)s]3 shown above, of Cav point group, consider the rotation axes: 2C4 and C2. If the axial CN ligand was substituted by CO, forming [Ni(CO)(CN)4]?, maintaining the square pyramidal geometry, which symmetry elements would be retained: i. Both C4 and C2. ii. Only the C2. iii. Only the C4. iv. None of the above b. Looking at the symmetry planes: 20v, 20d; what symmetry elements would be retained if the Co substitution would occur on an equatorial CN ligand (forming again [Ni(CO)(CN)4]?) instead? The dihedral planes in this case is the mirror plane that bisects the square planar bonds. i. All ov ii. All od iii. At least 1 od iv. At least 1 oyarrow_forwardChoose all chelating ligands from the substances below. OA. hydroxide ion B. ethylenediamine C. oxalate ion OD. oxide ionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY