
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Question 4: Based on the procedure provided, how much acetic acid (in mL) should Ashe use when setting up the reaction at this new scale (to make 0.750 g of benzopinacolone)?
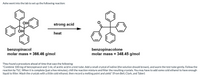
Transcribed Image Text:Ashe went into the lab to set up the following reaction:
OH
strong acid
OH
heat
benzopinacol
molar mass = 366.46 g/mol
benzopinacolone
molar mass = 348.45 g/mol
They found a procedure ahead of time that says the following:
"Combine 100 mg of benzopinacol and 1 mL of acetic acid in a test tube. Add a small crystal of iodine (the solution should brown), and warm the test tube gently. Follow the
reaction by TLC. When it is complete (just a few minutes), chill the reaction mixture and filter the resulting crystals. You may have to add some cold ethanol to have enough
liquid to filter. Wash the crystals with a little cold ethanol, then record a melting point and yield." (From Bell, Clark, and Taber)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Mixture 1 Reference 2 Extra Fe(NO3)3 3 Extra KSCN 4 Add Na₂SO3 5 Add NaCl 6 Add AgNO3 Volume (mL) | Volume (mL) of 0.002 M Fe(NO3)3 2.00 2.00 2.00 2.00 2.00 2.00 of 0.002 M Other Addition KSCN 2.00 2.00 2.00 2.00 2.00 2.00 N/A 0.2 mL of 0.1 M Fe(NO3)3 0.2 mL of 0.1 M KSCN Na₂SO3 NaCl 1 mL of 0.1 M AgNO3arrow_forwardUsing the solubility information from your table of constants, make a flow chart, showing the procedure you would use to separate 4- chloroaniline from 1,4-dibromobenzene. Indicate what compound is in each solvent by writing the structures at the appropriate locations in the flow chart. please answer the question, and draw the chart.arrow_forwardwhich procedure will produce 200ml of a solution that is 2.5%by volume alcohol of water?arrow_forward
- How would each of the following errors affect the experimental value of your molarity of acetic? Would your Molarity value be too high, too low, or unaffected? Explain your answer. if you titrated your acid sample to a bright pink rather than faint pink endpoint? if your "acetic acid" burette was still wet inside with deionized water when you filled it with acetic acid? if you recorded the final volume of NaOH as "39.88 mL" instead of "38.88 mL"?arrow_forwardWhat would the % yield of ester be if the reaction were simply allowed to equilibrate (i.e. no products are removed)? C3H7OH+C2H5COOH=C6H12O2+H2O Percent yield of ester was 71.6% weight of product= 20.7868g 19.0mL (0.25mol) of n- propanol 18.5 mL (0.25 mol) of propionic acid At room temperature, the equilibrium constant, Keq is approximately 3.arrow_forwardThe simplest method for showing a separation scheme is in flow chart form. The table below gives you an example of three cations (Pb2*, Cu2+, and Ba2) with various reagents. Construct a flow chart from the table for separating these three ions from each other. See page 9-10 of the CHEMV01BL lab manual for an example. You do NOT need to include balanced equations nor dissolve solids into aqueous solution before separating. Reagent Pb2+ Cu2+ Ba2 HCI white precipitate no reaction no reaction H2SO4 white precipitate no reaction white precipitatearrow_forward
- (extraction, reactive extraction, recrystallization). Write a few sentences for each technique explaining themarrow_forwardUsing the data tables provided, 1. Determine the molarity of NaOH for each of the three trials. Remember that NaOH and KHP (C8H5KO4) react in a 1:1 ratio 2. What is the average molarity of NaOH from the three trials 3. Determine the molarity of the H2SO4 for each of the three trials using the average molarity of NaOH that you calculated in #2arrow_forwardPlease help me answer these question 4. Does the pooling of results in this experiment yield an average % Mg in MgO, which is closer to the accepted value than your class average? 5. Compare the precision obtained by your class to the precision obtained by the composite of all classes. Discuss. 6. What conclusion can you draw from the analysis of the data in assessing whether this laboratory procedure gives accurate results?arrow_forward
- The KI used in this lab has a concentration of 0.500M. In trials 1-4, 1.0 ml of this solution is combined with 4.0 ml of the H2O2. What is the concentration of the KI in this solution after mixing but before the reaction occurs. ( Enter the result in table 1 on the report sheet) (2 sig figs)arrow_forwardSelect one for each boxarrow_forwardhow many equivalents of 6M NaOH would you add (in other words, what molar ratio of 6M NaOH to triester do you need) so that the reaction runs as pictured?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY