
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
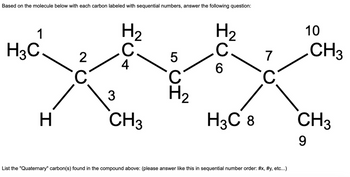
Transcribed Image Text:Based on the molecule below with each carbon labeled with sequential numbers, answer the following question:
1
H3C
H
2
с
H ₂
C.
4
3
CH3
5
ين
H₂
C
6
H3C 8
7
C
List the "Quaternary" carbon(s) found in the compound above: (please answer like this in sequential number order: #x, #y, etc...)
10
CH3
CH3
9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- a. Br₂(l) is [Select ] b. C8H18(l) is [Select] c. AgCl(s) is [Select] d. PCI (s) is [Select] Question 6 in C6H₂(1) A in C₂H6(1) in C6H6(1) ✓in CH₂(1) What is the mass percent of potassium chloride when 9.35 grams of potassium chloride is 162.98 grams of water? Choose the answer with the appropriate significant figures and carrow_forwardHow many moles of zinc are in 1.00 g? 20 E 4S D 1.00 g Zn = How many moles of krypton are in 1.00 g? 1.00 g Kr= M 888 R F 8.37 15 V 5 R JON C T G 6 B MacBook Pro Y 201 #tv H 7 N U 1 J PRI 8 A M MOSISO 9 K A : Aa ; M V option { +1 [ 134 ? mol Zn L mol Kr delete return shiftarrow_forwardPlease I need help with this question. Thanks.arrow_forward
- How many total atoms are in the compound CuSO4? How many elements are in 2H2SO4? How many molecules are found in this formula 2Li3PO4? How many atoms of carbon are found in glucose, C6H12O6?arrow_forwardPlease help me answer the subpart d, e and farrow_forwardHow many grams of ClCl are in a 38.0-g sample of the chlorofluorocarbon C2F3Cl3? Express your answer in grams of chlorine to three significant figures.arrow_forward
- Carvone (C10H14O) is the main component of spearmint oil. It has a pleasant aroma and mint flavor. Carvone is added to chewing gum, liqueurs, soaps, and perfume. If you had 55.4 g of carvone, how many carbon atoms do you have?arrow_forwardA 30.21 g sample of a compound that contains only carbon and hydrogen was combusted and yielded 105.18 g of CO₂ and 13.45 g of H₂O. The molecular mass of the compound is 202.2 amu. What is this compound's molecular formula? A) C₁₅H₂₂ B) C₈H₅ C) C₁₆H₁₀ D) C₁₆H₁₂arrow_forwardPls help ASAP. Pls help in all asked questions.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY