
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
How many C atoms?
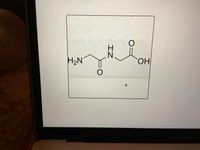
Transcribed Image Text:H2N
HOH
MacBook Pro
IZ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many atoms are present in 0.430 g of gold?arrow_forwardCalculate the mass in grams of 14 atoms of Ca.arrow_forwardElemental phosphorus occurs as tetratomic molecules, P4. What mass of chlorine gas is needed to react completely with 223 g of phosphorus to form phosphorus pentachloride? _____________ g Cl2arrow_forward
- How do the percent compositions for A C₂H6 and 48 compare? CH 36 C 48 has a higher percentage of carbon than C3H6. B CH CH C.Hs has a higher percentage of hydrogen than 36. C They are the same. D The percent compositions cannot be determined.arrow_forwardThe mass in grams of 2.0x10-2 moles of C6H12O6arrow_forward1. How many grams of Fe" are present in 3.42 grams of iron(II) sulfide? grams Fe2+. 2. How many grams of iron(II) sulfide contain 1.55 grams of Fe2*? grams iron(II) sulfide.arrow_forward
- How many molecules are in 119.78 g of butane (C4H10)?arrow_forwardWhich weighs less, 0.25 mole of xenon atoms or 2.0 moles of carbon atoms?arrow_forward12 of 3 What amount of CUSO4•5H,O, in moles, does this mass represent? mass = 1.787 g X mol resubmit Incorre The molar mass of CuSO4 5H,O is 249.69 g/mol.arrow_forward
- The amounts of two elements X and Y in three samples of an unknown substance are as follows: 10.0 g X and 10.0 g Y in Sample 1 18.0 g X and 12.0 g Y in Sample 2 6.0 g X and 2.0 g Y in Sample 3 What is this substance? Hint: Compare the percentages of Y (or X) in each sample. Are these percentages of Y the same or different? I will say that in sample 1 the percentage of Y is 50 %. likely a mixture likely a compound likely an element either a compound or an element, but cannot be a mixturearrow_forwardIf you have a 25% Ca in calcium fluoride, what is the mass of Ca if you have 2.40 g of calcium fluoride?arrow_forwardCalculate the following, with proper number of significant figures: Molar mass of Al(CH3COO)3 is ____________ g/mol In 16.4 moles of Al(CH3COO)3 … … the number of grams of Al(CH3COO)3 is ____________ g … the count of Al(CH3COO)3 units is ____________ … the number of moles of 6C atoms is ____________ mol … the count of 6C atoms is ____________ … the mass of 6C atoms is ____________ g The % mass fraction of 6C atoms in Al(CH3COO)3 is ____________% The % mass fraction of Al atoms in Al(CH3COO)3 is ____________% Name the compound: ____________arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY