
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Based on the Henderson-Hasselbalch equation (shown below), calculate the pH when half of a solution of acetic acid is dissociated to acetate (the pKa of acetic acid is 4.76).
A. 1.00
B. 3.76
C. 4.76
D. 5.76
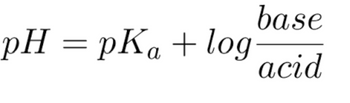
Transcribed Image Text:pH = pKa + log
base
acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Give answer of all parts with final answerarrow_forward3) Ammonia, NH3, is toxic to a wide range of aquatic organisms including snails, insects, and fish. The ammonium ion, NH4", is much less toxic since the cation does not diffuse rapidly across cell membranes. The US EPA limit for total ammonia in natural waters is 1.9 mg/L at pH 7. a. What is this limit in molar units? b. What is the concentration of NH3 at pH 7? c. What is the concentration of NH3 at pH 6? d. Why is the EPA limit for total ammonia dependent on the water pH?arrow_forwardWhat is the concentration in % (w/w), of a solution prepared by mixing 50.0 g NaCl and 150.0 g water? A. 0.250 % B. 33.3 % C. 40.0 % D. 25.0 % E. 3.00 %arrow_forward
- Give answer of all parts with final answerarrow_forwardA scientist extracted compounds from apple peel and separated them using gel exclusion chromatography. Indicate which would be the expected order of elution from first eluted to last.arrow_forward17. 1- Butanol was converted into butyl propanoate by reaction with an excess of propanoic acid. What is the name of this reaction? A. Acid-base reaction B. Base hydrolysis C. Acid hydrolysis D. Esterificationarrow_forward
- 8.52 Will a red blood cell undergo crenation, lysis, or no change in each of the following solutions? a. 10% (m/v) NaCl b. distilled water c. 5% (m/v) NaCl d. 5% (m/v) glucose e famsarrow_forwardThe pH of the amino acid shown below is:arrow_forwardHow many ml of a 40% solution can be prepared from 42.8 mg of sucrose? How many ml of a 40% solution can be prepared from 42.8 mg of sucrose? Question 3 options: b. 0.107 µl e. 1.09 µl a. 1.11 µl c. 10.08 µl d. 2.08 µlarrow_forward
- Define the following terms: a. arginosuccinate b. Krebs bicycle c. N-acetylglutamate d. BH4 e. taurinearrow_forwardBased on the Henderson-Hasselbalch equation (shown below), calculate the pH when the ratio of acetic acid to acetate is 10 to 1 (the pKa of acetic acid is 4.76). A. 1.00 B. 3.76 C. 4.76 D. 5.76arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON