
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Based on question 2 and 3 what combination of temperature and vinegar concentration would best achieve a slow oozing reaction
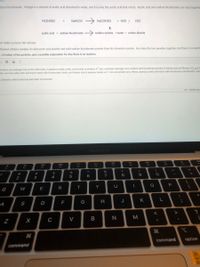
Transcribed Image Text:dium bicarbonate. Vinegar is a mixture of acetic acid dissolved in water, and it is only the acetic acid that reacts. Acetic acid and sodium bicarbonate can react togethe
HC2H302
NaHCO3
NAC2H302
+ H20 +
CO2
acetic acid + sodium bicarbonate
sodium acetate +water + carbon dioxide
mer make a science fair volcano,
Samuel obtains samples of solid acetic acid powder and solid sodium bicarbonate powder from his chemistry teacher. He mixes the two powders together, but there is no reacti
e of matter of the particles, give a possible explanation for why there is no reaction.
produce any hydrogen ions in the solid-state. In aqueous media, acetic acid lonizes to produce H ions, and these hydrogen ions combine with bicarbonate present in baking soda and liberate CO, gas.9
etic acid, but solid acetic acid never reacts with bicarbonate. Acetic acid ionizes only in aqueous media as H ions and acetate ions. Hence, aqueous acetic acid reacts with bicarbonate and liberates carbc
m between solid bicarbonate, and acetic acid powder.
86 / 10000 Wor
MacBook Air
888
FS
F3
&
23
6
7
8.
2
3
P
Y
Q
S
D
F
G
H
J
A
>
C
V
N
M
command
option
command
B
R
的 4
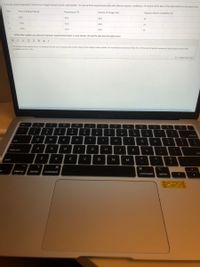
Transcribed Image Text:2. For his second experiment, Samuel uses vinegar instead of acetic acid powder. He sets up three experimental trials with different reaction conditions. He records all his data in the table below for the three trials.
Trial
Mass of Baking Soda (g)
Temperature ("C)
Volume of Vinegar (ml)
Reaction time to completion (s)
10.0
25.0
60.0
41
10.0
40.0
60.0
23
10.0
55.0
60.0
12
a) Describe a pattern you observe in Samuel's experimental results. In your answer, cite specific data from the table above.
BIVEE I A I
On analysis of the reaction trials, it is observed that the mass of baking soda and the volume of the vinegar is kept constant. On increasing the temperature from 25 to 55 the rate of reaction increases by reducing the reaction time
gradually from 41s to 12s.
47 /10000 Word Limit
https//student.masteryyconnect
GVsX2kjosNziwOTUSNSwic3R1ZOvudFudWZXioisMIYMz13wic3R1ZGVudr
MacBook Air
esc
%23
%24
&
3
5
6.
7
8
delete
Q
R
T.
Y
U
tab
caps lock
D
G
J
K
ret
shift
C
V
в
N
M
control
option
command
command
option
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- d) In 4 test tubes, divide equally the solution prepared in procedure c into four parts. Reserve the first test tube as a standard. 1) To the second test tube add 1 mL of ferric chloride solution. 2) To the third test tube add 1 mL of ammonium thiocyanate solution. 3) To the fourth test tube add a pack of solid ammonium chloride. Compare the color obtained in test tubes 2,3,4 with the first test tube(standard solution). Record and explain your observations. Was the reaction in procedure c complete? Can the reaction be made reversible? Define common ion effect. 2. a) Heat a pack of sawdust in a stained test tube. Observations. Is the reaction reversible or irreversible? Complete or incomplete? b) Recall the reactions of: i. Magnesium ribbon (ignited) and oxygen; ii. potassium chlorate and heat; iii. blue vitriol and heat. Write the chemical equations for each of the above reactions and classify each as reversible or irreversible and complete or incomplete?arrow_forwardIf 10.0 g of silver nitrate is available, what volume of 0.25 M silver nitrate can be prepared?arrow_forwardCreate a data table with the following columns: Column 1 Title: Molarity of Vinegar (Record this as 0.83 Molar) Column 2 Title: Volume of Vinegar Used Column 3 Title: Number of Tums Tablets Used (This should be 1)arrow_forward
- 75.0ml of 0.085 m potassium sulfate is added to 50.0mL of 0.055M tin (II) nitrate forming a solid product. Determine the number of moles of each reactant Determine the limiting reactant Determine the reactant in excessarrow_forward3. Which of these is the active ingredient in vinegar? A. calcium hydroxide B. sulfuric acid C. phosphoric acid D. ammonium phosphate E. acetic acidarrow_forwardIf you wish to prepare 1.5 liters of 0.30 M HCl solution from. A stock solution that has a concentration of 6.0 M, what volume of the 6.o M solution would be required?arrow_forward
- 13 982 72.5 50 in 69 1 Br 08.9 69 Tm 8.934 1No (259) 45 Chemistry I Laboratory Manual, 2017 Revision Questions: 1) Calculate the theoretical percent water in the following hydrates. (a) chromium(III) nitrate nonahydrate (b) tin(II) chloride dihydrate the lowing reaction will occurarrow_forwardHow do I create a graph, using my table. Can you please do an example or explain. Thank You I have to show thats it is intersecting like on the example.arrow_forwardHow many mL of 0.300 M NaCl solution are required to produce 0.115 moles of NaCl?arrow_forward
- please answer Question 7arrow_forwardYou need to make an aqueous solution of 0.138 M manganese(II) bromide for an experiment in lab, using a 500 mL volumetric flask. How much solid manganese(II) bromide should you add? gramsarrow_forward__________ is the solvent in an aqueous solution of sodium sulfate and barium nitrate. barium nitrate sodium sulfate water barium sulfate sodium nitratearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY