
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:困
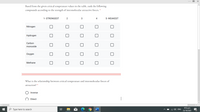
Based from the given critical temperature values in the table, rank the following
compounds according to the strength of intermolecular attractive forces. *
1- STRONGEST
2
3
4
5- WEAKEST
Nitrogen
Hydrogen
Carbon
monoxide
Охудen
Methane
What is the relationship between critical temperature and intermolecular forces of
attraction? *
Inverse
O Direct
|
4:52 pm
P Type here to search
a 4) ENG
23/10/2021
O O
O O O
近
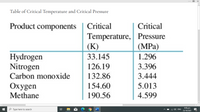
Transcribed Image Text:Table of Critical Temperature and Critical Pressure
Product components
Critical
Critical
Temperature, Pressure
(К)
(MPa)
1.296
Hydrogen
Nitrogen
Carbon monoxide
33.145
126.19
3.396
132.86
3.444
Охygen
154.60
5.013
Methane
190.56
4.599
4:48 pm
O Type here to search
a 1) ENG
23/10/2021
近
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- 5-25 A gas in a bulb as in Figure 5-3 registers a pressure of 833 mm Hg in the manometer in which the reference arm of the U-shaped tube (A) is sealed and evacuated. What will the difference in the mercury levels be if the reference arm of the U-shaped tube is open to atmospheric pressure (760 mm Hg)?arrow_forwardOne of the chemical controversies of the nineteenth century concerned the element beryllium (Be). Berzelius originally claimed that beryllium was a trivalent element (forming Be3+ ions) and that it gave an oxide with the formula Be2O3. This resulted in a calculated atomic mass of 13.5 for beryllium. In formulating his periodic table, Mendeleev proposed that beryllium was divalent (forming Be2+ ions) and that it gave an oxide with the formula Be2O3. This assumption gives an atomic mass of 9.0. In 1894, A. Combes (Comptes Rendus 1894, p. 1221) reacted beryllium with the anion C5H7O2and measured the density of the gaseous product. Combess data for two different experiments are as follows: I II Mass 0.2022 g 0.2224 g Volume 22.6 cm3 26.0 cm3 Temperature 13C 17C Pressure 765.2 mm Hg 764.6 mm If beryllium is a divalent metal, the molecular formula of the product will be Be(C5H7O2)2; if it is trivalent, the formula will be Be(C5H7O2)3. Show how Combess data help to confirm that beryllium is a divalent metal.arrow_forwardIn dry atmospheric air, the four most abundant components are N₂, X = 0.808; O₂, X = 0.1915; Ar, X = 9.34x10-5; and CO₂, X = 4.066x104. Calculate the partial pressure of CO₂, in torr, under standard atmospheric conditions. Submit Answer Tries 0/99 This discussion is closed. M CAL 8:00- 10:50- CI 12:00 Q ! 2 @ W OCT 21 F2 # 3 E 80 F3 $ 4 R 10 Q F4 % 5 T F5 A 6 Y F6 tv A S MacBook Air & 7 8 F7 8 DII FB ( 9 F9 O ) 0 J F10 F11 + F12 Send Feedbackarrow_forward
- Determination of the Universal Gas Constant Postlab 1. An evaluation of Rwas performed using the procedures outlined in this exercise, The barometric pressure was 735 torr and the temperature was 21.5 "C. The volume of H2(9) collected was 32.7 mL. The calculated value of Rwas 0.0817 L'atm/mol-K. a. How many grams of Mg metal were used in this determination? 0.0316 70.361 b. If the correction for the vapor pressure of water had not been performed, what would be the calculated value of R? Incorrect c. If the syringe volume had been incorrectly read, resulting in a calculated H, volume of 30.6 mL, what would be the percent error in the calculated value of Rwith respect to the originally calculated value (0.0817 L-atm/mol·K)? 6.4 %arrow_forwardThe student from question 3 then heated the beaker of water and found that at 75 °C the corrected volume of the bubble was 4.05 mL. What should she report as the vapor pressure of water PH2O) at this temperature? Answer from question 3= 0.000095 moles of trapped air This was also the question for 3 just in case you needed it for information: A student found that at 755 torr atmospheric pressure and 0.2 °C, the corrected volume of the trapped air was 2.15 mL. Under these conditions, how many moles of trapped air nair are present?arrow_forwardIn Part B, you used a similar procedure to that of Part A to determine the mass percent of CaCO3 in an antacid tablet. The commercially accepted range of CaCO3 in an antacid tablet is 25–35% by mass. Miranda obtained the data below from her Part B trial: TRIAL PRESSURE (torr) MASS OF TABLET (g) TEMPERATURE (°C) VOLUME CO2 RECOVERED (mL) 1 750.5 0.5702 21.3 69.6 2 750.5 0.5622 21.2 68.2 Miranda determined her average % Recovery of CO2 to be 65.0% from Part A. Based on her data collected from Part A, was the average percent mass of CaCO3 in her antacid tablet within the commercially accepted range? Yes, her mass percent does fall within the commercially accepted range. No, her mass percent does not fall within the commercially accepted range.arrow_forward
- Water Vapor pressure at 23c is 21.7 mmHGarrow_forwardThank youarrow_forwardom.google.com/u/1/w/Mzg1MzgyNTkwMDQy/t/all Partial Pressure Assessment 85. Consider the flasks in the following diagram. What are the fi- nal partial pressures of H, and N, after the stopcock between the two flasks is opened? (Assume the final volume is 3.00 L.) What is the total pressure (in torr)? 2.00 L H- 1.00 L N, 475 torr 0.200 atm. (1 atm- 760 torr) Pagearrow_forward
- Composition of Dry Air at Sea Level Gas Mole% N2 78.084 O2 20.946 Ar 0.934 CO2 0.0345 Ne 0.00182 He 0.000524 CH4 0.000168 Kr 0.000114 Using data from the above table, calculate the partial pressure of CO2 when the atmospheric pressure is 1.06 atm.At the same atmospheric pressure, if the temperature is 10°C, how many CO2 molecules would there be in 850 mL?arrow_forwardApplications of physical chemistry to daily lifearrow_forwardPlease solve sub partsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning