
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:ball & stick
+ labels
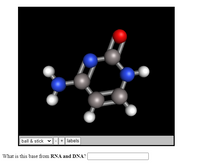
What is this base from RNA and DNA?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ball & stick ♥ + labels What is this base from RNA and DNA?arrow_forward3. For this short DNA segment, a. identify the 5' end and the 3' end of the molecule. b. circle the atoms that comprise the backbone of the nucleic acid chain. c. write the nucleotide sequence of this DNA segment. HN o=P-OCH, CH, NH o=P-OCH, HN o=P-OCH, ноarrow_forwardWhat effect do restriction enzymes have on DNA? Select one: Restriction enzymes repair damaged portions of DNA. O Restriction enzymes cleave DNA at specific sequences into fragments. Restriction enzymes give a negative charge to DNA. Restriction enzymes duplicate DNA.arrow_forward
- 0 0=P-0-CH₂ 0- H 1) How would you designate this acid in a abbreviation ? 09-0-962 6- H 3) Provide -NH₂ one-letter AR # # 2) 15 this a strand of DNA of RNA? How do you know? an Ntl₂ 0=P-O-CH₂ A K он н abbreviation for the Strand of DNA WITH complementary bases to the one shown above. Indicate the '5' and 3 endsarrow_forwardThe genetic information contained in a DNA molecule is ultimately expressed in a. an mRNA molecule b. an rRNA molecule c. the structure of a protein d. the transcription of the DNAarrow_forward1. Which of the following is not a component of a nucleic acid? a. phosphate group b. Amino acid c. Sugar d. Nitrogen containing base e. All are components of a nucleic acid 2. What is the name of the process of using messenger RNA sequences for encoding for proteins synthesis in the ribosomes? a. Transcriptionb. duplication c. replication d. Translation e. Cloning 3. Which of the following is not a characteristic of RNA? a. Uracil is one of the bases b. RNA is single stranded c. The sugar is 2-'Deoxyribose d. There are three types of RNA e. adenine and uracil form a base pairarrow_forward
- Which nucleic acid is formed during transcription? a. DNA b. rRNA c. tRNA d. mRNAarrow_forwardDraw an example of the followings: 1- an amino acid; 2- a small peptide; 3- a DNA nucleotide; 4- an RNA nucleotide.arrow_forwardDraw the structure of base pairing between 2 compliantly bases. In DNA. Show H bonding.arrow_forward
- 1. Draw the two Watson-Crick Bases Pairs and show how G- C and A-T recognize each other by hydrogen bonding. 2. Draw the full structure of two nucleotides - one from DNA and one from RNA. Point out the key difference between the two. 3. Write out an example of a condensation reaction forming a phosphate-sugar bond. 4. Write out an example of a condensation reaction between a nucleic acid base and a sugar. 5. State the "Central Dogma" of biochemistry.arrow_forwardDeoxyadenosine monophosphate (dAMP) and guanosine monophosphate (GMP) are nucleotides. The similarities between dAMP and GMP are that they both have? -an alpha (central) carbon.-the same R group.-a phosphate group.-a pentose (5 sided) sugar-an amino group-a nitrogenous base.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY