
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
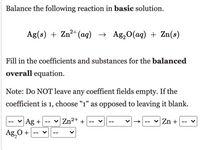
Transcribed Image Text:Balance the following reaction in basic solution.
Ag(s) + Zn²+(aq) → Ag,O(ag) + Zn(s)
Fill in the coefficients and substances for the balanced
overall equation.
Note: Do NOT leave any coeffient fields empty. If the
coefficient is 1, choose "1" as opposed to leaving it blank.
-- v Ag + --
v Zn2+ +
v
Zn +
Ag,0 +
-- v--
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Balance the following equations. When balancing equations for redox reactions in aqueous solution, it is necessary to add water molecules (H₂O) and either H+ (aq) in acidic solution or OH(aq) in basic solution to the equation. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank. If needed, use H+ for the hydronium ion.) a. b. Submit Answer 2+ Zn(s) + VO²+ (aq) + + + Ni(s) + CIO¯¯ (aq) Ni(OH)2 (s) + Cl(aq) (basic solution) + → Zn²+ (aq) + V³+ (aq) (acid solution) Retry Entire Group + + + 6 more group attempts remainingarrow_forwardHelparrow_forwardFor the following redox reactions, assume that each ion concentration is 0.10 M. For which reaction is Ecell < Eocell? Group of answer choices 2 Na(s) + Cu2+ → 2 Na+ + Cu(s) Zn(s) + Pb2+ → Zn2+ + Pb(s) Cr(s) + Au3+ → Cr3+ + Au(s) Mn(s) + 2 K+ → Mn2+ + 2 K(s)arrow_forward
- Balance the following reaction in acidic solution. Fe?+ (ag) + MnO, (aq) → Fe+ (ag) + Mn²+(aq) Fill in the coefficients and substances for the balanced overall equation. Note: Do NOT leave any coeffient fields empty. If the coefficient is 1, choose "1" as opposed to leaving it blank. Fe2+ + MnO,¯ + 4 Fe3+ + Mn2+ +arrow_forwardIn the Redox reaction shown Fe(s) + CuCl2(aq) -------> Cu(s) + FeCl2(aq) O A. Fe, Fe²+ OB. Cu, Cu²+ OC. Cu2+, Cu OD. Fe Fe¹+ is oxidized and becomesarrow_forwardbalance the chemical equation for each reaction C7H602 + 02-----> H20 + C02arrow_forward
- 1. Cu(NO3)2(aq)+2NaOH(aq) to Cu(OH)2(s)+2NaNO3(aq) 2. Cu(OH)2(s) to CuO(s)+H20(l) is this a single replacement double combination decomposition combustionarrow_forwardBalance the reaction between Fe and HNO3 to form Fe2+ and NO in acidic solution. When you have balanced the equation using the smallest integers possible, enter the coefficients of the species shown. Fe + HNO3 Fe2+ + NO (reactant, (Enter 0 for neither.) Water appears in the balanced equation as a product, neither) with a coefficient of How many electrons are transferred in this reaction?arrow_forwardi don’t know what it is asking forarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY