
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
14) Balance the following
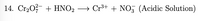
Transcribed Image Text:**Chemical Reaction Equation in an Acidic Solution**
14. \( \text{Cr}_2\text{O}_7^{2-} + \text{HNO}_2 \rightarrow \text{Cr}^{3+} + \text{NO}_3^{-} \) (Acidic Solution)
This equation represents a chemical reaction occurring in an acidic solution, where dichromate ions (\(\text{Cr}_2\text{O}_7^{2-}\)) react with nitrous acid (\(\text{HNO}_2\)) to produce chromium ions (\(\text{Cr}^{3+}\)) and nitrate ions (\(\text{NO}_3^{-}\)).
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Is the reaction of zinc and sulfur a redox reaction? Write a complete, balanced chemical equation.If yes, show how you know this, including oxidation number determination show and identify the half reactions?arrow_forward2. Write the half-reaction equation for the oxidation of (a) NOz to NOs (acidic) Atoms: Oxygen: Hydrogen: Electrons: Was this half reaction oxidation or reduction?arrow_forwardexplain if further oxidation can occur.arrow_forward
- Im not really sure how or what I'm supposed to do for the question in the first picture with the flowchart, the second picture which is question #1 describes the need information for me to complete the one I'm stuck onarrow_forwarda) Write a balanced chemical equation describing the oxidation of chlorine gas by the copper(III) ion to form the chlorate ion and copper(II) in an acidic aqueous solution. Use the smallest whole-number coefficients possible. b) How many electrons are transferred in the redox reaction?arrow_forwardD) Oxidation-Reduction Reactions (Single Replacement) 7) Hydrogen is released when aluminum reacts with hydrochloric acid. 8) Magnesium reacts with silver nitrate solution.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY