
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Using the half-reaction method and showing all of your steps, write the balanced equation for
the
Be sure to show each step when balancing each half-reaction, as well as how the electrons
balance between the two half-reactions.
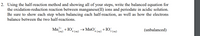
Transcribed Image Text:2. Using the half-reaction method and showing all of your steps, write the balanced equation for
the oxidation-reduction reaction between manganese(II) ions and periodate in acidic solution.
Be sure to show each step when balancing each half-reaction, as well as how the electrons
balance between the two half-reactions.
Mn* +I0,
4 (aq)
→ MnO,
4 (аq)
+ IO.
3 (аq)
(unbalanced)
(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each of the following, determine the half- reactions and net reaction and the minimum voltage required. 1. An aqueous solution of potassium sulfate is electrolyzed.arrow_forwardYou are tasked with creating a pourboix diagram of sulfur speciation. The relevent species are SO42-, HSO42-, S, H2S, and HS-. The total copper is 10^-6 mol. Set and balence the redox reactions needed for this question and explain how you landed on those reactions.arrow_forwardGive detailed Solution with explanation needed...give answer Step by step solution..give answer both sub parts if you not then don't give answerarrow_forward
- I need number 3 answers and conclusionsarrow_forward1. With reference to the following list, which of the statements is true? The following is a list of some of the metals in the galvanic series in their relative order: - Anode end - Tin - Zinc - Copper - Aluminium - Nickel - Cadmium - Titanium - Mild Steel - Platinum - Cast Iron - Cathode End ¨ copper is more corrodible than mild steel if they are in contact ¨ aluminium and nickel together have greater corrosion potential than aluminium and tin ¨ platinum is the most corrodible metal ¨ zinc is the least corrodible metalarrow_forward[Review Topics] [References] Use the References to access important values if needed for this question. Fe2+ + Ni→Ni²* + Fe In the above redox reaction, use oxidation numbers to identify the element oxidized, the element reduced, the oxidizing agent and the reducing agent. name of the element oxidized: name of the element reduced: formula of the oxidizing agent: formula of the reducing agent: Submit Answer Retry Entire Group 6 more group attempts remaining Previous Next Email Instructor Save andarrow_forward
- Nhen zinc metal is added to a beaker of hydrochloric acla, a gas is produced. Write the balanced net ionic equation for this reaction. Write the oxidation and reduction half reactions. What quantity of useful work can be obtained when Zn is added directly to the beaker of HCI? How can you harness this reaction to do useful work? Draw a diagram that shows this.arrow_forwardWrite the half-reaction that occurs at the anode during the electrolysis of NaCl. Include physical states and use single arrow in your equation. Incorrect. Recall that reduction takes place at the cathode, so the substance must be gaining electrons. Write the half-reaction that occurs at the cathode during the electrolysis of NaCl. Include physical states and use single arrow in your equation. Incorrect. Is your reaction balanced? There should be no electrons in a balanced final equation. Write the overall cell reaction for the electrolysis of NaCl. Include physical states and use single arrow in your equation.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY