
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
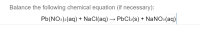
Transcribed Image Text:Balance the following chemical equation (if necessary):
Pb(NO:)2(aq) + NaCl(aq) → PbCl2(s) + NaNOs(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the chemical reaction HClO4(aq)+NaOH(aq)⟶H2O(l)+NaClO4(aq) write the net ionic equation, including the phases. Which ions are considered spectator ions for this reaction?arrow_forwardConsider the chemical reaction. 3 MgCl₂ (aq) + 2 Na₂PO₂ (aq) → Mg3(PO4)₂ (s) + 6 NaCl(aq) Write the net ionic equation for the chemical reaction, including phases.arrow_forwardYou have 0.765 g of an unknown acid, H₂A, which reacts with NaOH according to the balanced equation H₂A(aq) + 2 NaOH(aq) → Na2 A(aq) + 2 H₂O(l) If 25.78 mL of 0.461 M NaOH is required to titrate the acid to the second equivalence point, what is the molar mass of the acid? Molar mass = g/molarrow_forward
- How can we calculate the molarity of the solution?arrow_forwardChlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, HCl(aq), as described by the chemical equation MnO₂ (s) + 4 HCl(aq) →MnCl₂(aq) + 2 H₂O(1) + Cl₂(g) How much MnO₂ (s) should be added to excess HCl(aq) to obtain 295 mL Cl₂(g) at 25 °C and 715 Torr? and X la # mass of MnO₂: 3 E D C $ 4 R F 675 % V T Garrow_forwardWhat mass of precipitate (in g) is formed when 20.5 mL of 0.500 M Cu(NO₃)₂ reacts with 29.0 mL of 0.500 M NaOH in the following chemical reaction? Cu(NO₃)₂(aq) + 2 NaOH(aq) → Cu(OH)₂(s) + 2 NaNO₃(aq)arrow_forward
- Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, HCl(aq), as described by the chemical equation MnO, (s) + 4 HCI(aq) – MnCl, (aq) + 2 H,0(1) + Cl, (g) > How much MnO, (s) should be added to excess HCl(aq) to obtain 105 mL Cl, (g) at 25 °C and 755 Torr? mass of MnO,:arrow_forwardWhat mass of precipitate (in g) is formed when 20.5 mL of 0.500 M Cu(NO₃)₂ reacts with 26.0 mL of 0.500 M NaOH in the following chemical reaction? Cu(NO₃)₂(aq) + 2 NaOH(aq) → Cu(OH)₂(s) + 2 NaNO₃(aq)arrow_forwardwhat is the balance chemical equation, complete ionic equation, and net ionic equation for NaOH (aq) + HCl (aq)? what is the balance chemical equation, complete ionic equation, and net ionic equation for BaCl2 (aq) +H2SO4 (aq)? what is the balance chemical equation, complete ionic equation, and net ionic equation for NH4OH (aq) + H2SO4 (aq)?arrow_forward
- In the following chemical reaction, which element is the reducing agent? 3 Sn(NO₃)₂(aq) + 2 Fe(s) → 2 Fe(NO₃)₃(aq) + 3 Sn(s)arrow_forwardWhat mass of precipitate (in g) is formed when 20.5 mL of 0.500 M Cu(NO₃)₂ reacts with 23.0 mL of 0.500 M NaOH in the following chemical reaction? Cu(NO₃)₂(aq) + 2 NaOH(aq) → Cu(OH)₂(s) + 2 NaNO₃(aq)arrow_forwardWrite the net ionic equation and the total ionic equation for Ca(OH)2(s) +2HCl(aq) = CaCl2(aq) +2H2O(l)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY