
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
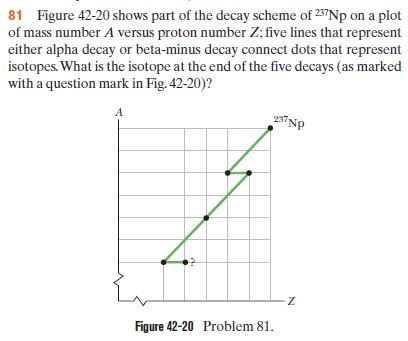
Transcribed Image Text:81 Figure 42-20 shows part of the decay scheme of 257NP on a plot
of mass number A versus proton number Z; five lines that represent
either alpha decay or beta-minus decay connect dots that represent
isotopes. What is the isotope at the end of the five decays (as marked
with a question mark in Fig. 42-20)?
237NP
Figure 42-20 Problem 81.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- e) The nucleus16 B(Z=5)has not been detected in nuclear reaction studies. Explain why. (Please type answer no write by hend).arrow_forwardThe most common isotope of uranium 23 U has a half-life of 4.47 x 10⁹ years, decaying to 92 234 Th by a emission. i) ii) iii) iv) Write down the nuclear decay equation. What is meant by the decay constant? Calculate the mass of uranium would be required for an activity of 6.00 µμCi. Calculate the number of a particle emitted per second by 30.0 g of uranium. [1 Ci = 3.70 x 10¹⁰ decays/s]arrow_forwardBased on data acquired during a radioactive decay lab using an unusual assortment of geometrically novel dice, the following trendline equation for an R vs. rolls graph is obtained: y = 321.03e-0.41x. Determine the decay constant, mean life, half-life, Nor & Ro. decay constant = mean life = half-life = R₂ = No = rolls rolls decays/roll dice rolls-1arrow_forward
- 15. A nucleus with 96 protons (and 248 nucleons) is bombarded with a neutron. Two products emerge from the collision. The first product is a proton. What is the second product? A. A nucleus with 95 protons B. A nucleus with 96 protons C. A nucleus with 151 neutrons D. A nucleus with 152 neutronsarrow_forwardProblems:1. Living matter has 1.3 x 10-10 % of its carbon in the form of C-14 which has a half-life of 5730 yr. A mammoth bone has a 300-g sample of carbon separated from it, and the sample is found to have an activity of 20 decays per second. How old is the bone?arrow_forwardRadon gas has a half-life of 3.83 days. If 2.92 g of radon gas is present at time t = 0, what mass of radon will remain after 2.20 days have passed? garrow_forward
- c) Complete the following decay reaction to show sodium-24 undergoing ߯ decay. First identify the unknown daughter nucleus (?).. and then name the subatomic particle that completes the reaction. 24Na → (?) + v + (subatomic particle) Enter the mass number A = Enter the atomic number Z = Enter the chemical symbol: Name the subatomic particle: A/ Narrow_forwardPart A A sample of U (T1= 1.59 × 10° yr) contains 2 233 6.10 x 1018 nuclei. What is the decay constant? Express your answer using three significant figures. ? = s-1 IIarrow_forwardA nuclear/ radioactive decay may be best expressed as Group of answer choices N = No e-1/λλt No = N e-λλt N = No e-λλt, where the symbols have their own significances N = No eλλtarrow_forward
- An unlabeled container of radioactive material has an activity of 78 decays/min. Four days later the activity is 63 decays/min. A. Determine the half-life of the material. T = ? days B. How many days after the activity is 78 decays/min will it reach 10 decays/min? t = ? daysarrow_forwardB3. In the alpha decay of uranium 232U → 228Th + He, how much kinetic energy (in MeV) will 90 the thorium atom have. Given that Q = 6.46 MeV and the atomic mass of 220Th is 228.028731u. Express the values to 3 significant figures, if necessary.arrow_forwardWhat is the nuclear equation for Th (beta decay)? (Just enter a, b, c or d without a period). 90 235 a. 334TH 90 b. 234 Th 234 Ac + e 90 89 4 Pa + °1e 234 c. 30 Th d. 84TH - 1 Pa + °e 90 O a O b O c « Previous MacBook Proarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON