
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:(VI) reacted with thionyl chloride in the presence of pyridine.
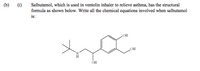
Transcribed Image Text:(b)
(i)
Salbutamol, which is used in ventolin inhaler to relieve asthma, has the structural
formula as shown below. Write all the chemical equations involved when salbutamol
is:
HO.
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (A) H2 (B) H2 HCI - (C) H2 Consider the reactions shown: What type of reaction is it? Which is the correct product A, B or C Name the first reactant Name the productarrow_forwardConsider an aqueous solution of Na,COa that contains 2.5467 g Na,CO, (M.M. 105.99) dissolved in 250.0 mL solution. Which of the following statements is correct? A The solute is NazCO3 while the solvent is H2O. The solute is Na2CO3 but the solvent was not defined. The solvent is NazCO3 and the solute is water. The solution is Na,CO3 and the solvent is water.arrow_forwardConstants I Periodic Table Lakes that have been acidified by acid rain (HNO3 and H2S04 can be neutralized by a process called liming, in which limestone (CaCO3) is added to the Part A acidified water. What mass of limestone (in kg) would be required to completely neutralize a 15.1 billion-liter lake that is 1.9x10-5 M in H2SO4 and 8.5x10-6 M in HNO3? Express your answer using two significant figures. kg Submit Request Answer Provide Feedback Next >arrow_forward
- (NH,)OH HCI sodium fluoride lithium bromide ammonium sulfate calcium nitrate magnesium phosphate potassium carbonatearrow_forwardWrite a balanced chemical equation based on the following description:solid C₄H₁₀O is burned with oxygen gas to produce gaseous carbon dioxide and water vaporarrow_forwardPredict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is balanced and contains state symbols after every reactant and product. HNO, (aq) + H2O(1) ローロ Xarrow_forward
- Aspirin can be prepared from salicylic acid ( C7H6O3 ), which has a molar mass of 138.12 g/mol, and acetic anhydride ( C4H6O3), which has a molar mass of 102.04 g/mol. The density of acetic anhydride is 1.082 g/mL. C,H6O3 + C4HO3 → C3H3O4 + C2H3O2 What is the theoretical yield, in grams, of aspirin (C,H3O4), which has a molar mass of 180.15 g/mol, possible when reacting 3.01 g of salicylic acid with 3.73 mL of acetic anhydride? Type answer:arrow_forwardHydrogen is manufactured on an industrial scale by this sequence of reactions: CH, (g) + H,0 (g) CO (g)+3 H, (g) K1 CO (g) + H,0 (g)= CO, (g)+H, (g) K2 The net reaction is: CH4 (g) +2 H,0 (g) CO, (g)+4H, (g) K Write an equation that gives the overall equilibrium constant K in terms of the equilibrium constants K, and K,. If you need to include any physical constants, be sure you use their standard symbols, which you'll find in the ALEKS Calculator. Karrow_forwardHydrogen sulfide, H2S, is produced during decomposition of organic matter. When 0.4190 mol H2S burns to produce SO2 (9) and H20(g), -217.0 kJ of heat is released. What is this heat in kilocalories? Heat = kcalarrow_forward
- Colorful fireworks often involve the decomposition of barium nitrate and potassium chlorate and the reaction ofthe metals magnesium, aluminum, and iron with oxygen.(a) Write the formulas of barium nitrate and potassium chlorate.(b) The decomposition of solid potassium chlorate leads to the formation of solid potassium chloride and diatomic oxygen gas. Write an equation for the reaction.(c) The decomposition of solid barium nitrate leads to the formation of solid barium oxide, diatomic nitrogen gas, and diatomic oxygen gas. Write an equation for the reaction.(d) Write separate equations for the reactions of the solid metals magnesium, aluminum, and iron with diatomic oxygen gas to yield the corresponding metal oxides. (Assume the iron oxide contains Fe3+ ions.)arrow_forwardCombustion of hydrocarbons such as pentane (C5H₁2) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. 2. Suppose 0.440 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 18.0 °C. Calculate the volume of carbon dioxide gas that is produced. Round your answer to 3 significant digits. ローロ X 00 0°arrow_forwardCombustion of hydrocarbons such as hexane (C6H₁4) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid hexane into gaseous carbon dioxide and gaseous water. 2. Suppose 0.320 kg of hexane are burned in air at a pressure of exactly 1 atm and a temperature of 15.0 °C. Calculate the volume of carbon dioxide gas that is produced. Be sure your answer has the correct number of significant digits. OL X x10 Ś 18 Ararrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY