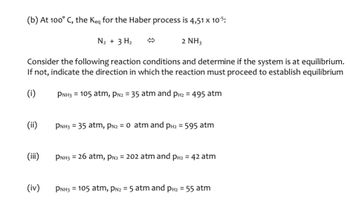
Problem 1ALQ: Consider an equilibrium mixture of four chemicals (A. B. C. and D. all gases) reacting in a closed... Problem 2ALQ: The boxes shown below represent a set of initial conditions for the reaction: Draw a quantitative... Problem 3ALQ: For the reaction H2+I22HI, consider two possibilities: (a) you add 0.5 mole of each reactant, allow... Problem 4ALQ: Given the reaction A+BC+D, consider the following situations: a. You have 1.3 M A and 0.8 M B... Problem 5ALQ: Consider the reaction A+BC+D. A friend asks the following: “I know we have been told that if a... Problem 6ALQ Problem 7ALQ: The value of the equilibrium constant, K, is dependent on which of the following? (There may be more... Problem 8ALQ: You are browsing through the Handbook of Hypothetical Chemistry when you come across a solid that is... Problem 9ALQ: What do you suppose happens to the Ksp, value of a solid as the temperature of the solution changes?... Problem 10ALQ: . Consider an equilibrium mixture consisting of H2O(g), CO(g). H2(g), and CO2(g) reacting in a... Problem 11ALQ: . Equilibrium is microscopically dynamic but macroscopically static. Explain what this means. Problem 12ALQ: In Section 17.3 of your text, it is mentioned that equilibrium is reached in a closed system. What... Problem 13ALQ Problem 14ALQ: . Consider the figure below in answering the following questions. a. What does a catalyst do to a... Problem 1QAP: For a chemical reaction to take place, some or all chemical bonds in the reactants must break, and... Problem 2QAP: For the simple reaction 2H2(g)+O2(g)2H2O(l)list the types of bonds that must be broken and the types... Problem 3QAP: How do chemists envision reactions taking place in terms of the collision model for reactions? Give... Problem 4QAP: When molecules collide, a certain minimum energy called the _________ is needed for the reaction to... Problem 5QAP: How does a catalyst work to speed up a chemical reaction? Problem 6QAP: Why are enzymes important? For example, what is the importance of the enzyme carbonic anhydrase in... Problem 7QAP: How does equilibrium represent the balancing of opposing processes? Give an example of an... Problem 8QAP: Consider the equilibrium process depicted in Fig. 17.6. When does the equilibrium state occur? Problem 9QAP: When writing a chemical equation for a reaction that comes to equilibrium. how do we indicate... Problem 10QAP: . How do chemists recognize a system that has reached a state of chemical equilibrium? When writing... Problem 11QAP: . What does it mean to say that a state of chemical or physical equilibrium is dynamic? Problem 12QAP: . Consider an initial mixture of N2 and H2 gases that can be represented as follows: The gases react... Problem 13QAP: . In general terms. what does the equilibrium constant for a reaction represent? What is the... Problem 14QAP: . There is only one value of the equilibrium constant for a particular system at a particular... Problem 15QAP: . Write the equilibrium expression for each of the following reactions. a.... Problem 16QAP: . Write the equilibrium expression for each of the following reactions. a. N2(g)+3Cl2(g)2NCl3(g)b.... Problem 17QAP: . Write the equilibrium expression for each of the following reactions. a.... Problem 18QAP: . Write the equilibrium expression for each of the following reactions. a. CO(g)+2H2(g)CH3OH(g)b.... Problem 19QAP: . Suppose that for the reaction PCl5(g)PCl3(g)+Cl2(g)it is determined, at a particular temperature.... Problem 20QAP Problem 21QAP: . At high temperatures, elemental nitrogen and oxygen react with each other to form nitrogen... Problem 22QAP: . Suppose that for the reaction 2N2O(g)+O2(g)4NO((g))it is determined, at a particular temperature,... Problem 23QAP: . What is a homogeneous equilibrium system? Give an example of a homogeneous equilibrium reaction.... Problem 24QAP Problem 25QAP: . Write the equilibrium expression for each of the following heterogeneous equilibria. a.... Problem 26QAP: . Write the equilibrium expression for each of the following heterogeneous equilibria. a.... Problem 27QAP: . Write the equilibrium expression for each of the following heterogeneous equilibria. a.... Problem 28QAP Problem 29QAP: . In your own words, describe what Le Châtelier’s principle tells us about how we can change the... Problem 30QAP: . Consider the reaction 2CO(g)+O2(g)2CO2(g)Suppose the system is already at equilibrium, and then an... Problem 31QAP: . For an equilibrium involving gaseous substances, what effect, in general terms, is realized when... Problem 32QAP: . What is the effect on the equilibrium position if an exothermic reaction is carried out at a... Problem 33QAP: . For the reaction system C(s)+H2O(g)H2(g)+CO(g)which has already reached a state of equilibrium,... Problem 34QAP Problem 35QAP: . Suppose the reaction system CH4(g)+2O2(g)CO2(g)+2H2O(l)has already reached equilibrium. Predict... Problem 36QAP: . Consider the general reaction 2A(g)+B(s)C(g)+3D(g)H=+115kJ/molwhich has already come to... Problem 37QAP: . Hydrogen gas and chlorine gas in the presence of light react explosively to form hydrogen chloride... Problem 38QAP: . Hydrogen gas, oxygen gas, and water vapor are in equilibrium in a closed container. Hydrogen gas... Problem 39QAP: . The reaction C2H2(g)+2Br2(g)C2H2Br4(g)is exothermic in the forward direction. Will an increase in... Problem 40QAP: . Old fashioned “smelling salts” consist of ammonium carbonate, (NH4)2CO3. The reaction for the... Problem 41QAP: . Plants synthesize the sugar dextrose according to the following reaction by absorbing radiant... Problem 42QAP: . Consider the exothermic reaction CO(g)+2H2(g)CH3OH(l)Predict three changes that could be made to... Problem 43QAP: . Suppose are action has the equilibrium constant K=1.3108. What does the magnitude of this constant... Problem 44QAP: . Suppose a reaction has the equilibrium constant K=1.7108at a particular temperature. Will there be... Problem 45QAP: . For the reaction Br2(g)+5F2(g)2BrF5(g)the system at equilibrium at a particular temperature is... Problem 46QAP: . Consider the reaction SO2(g)+NO2(g)SO3(g)+NO(g)Suppose it is found at a particular temperature... Problem 47QAP: . For the reaction 2CO(g)+O2(g)2CO2(g)it is found at equilibrium at a certain temperature that the... Problem 48QAP: . For the reaction CO2(g)+H2(g)CO(g)+H2O(g)the equilibrium constant. K, has the value 5.21103at a... Problem 49QAP: . The equilibrium constant for the reaction H2(g)+F2(g)2HF(g)has the value 2.1103at a particular... Problem 50QAP: . For the reaction 2H2O(g)2H2(g)+O2(g)K=2.4103at a given temperature. At equilibrium ills found that... Problem 51QAP: . For the reaction 3O2(g)2O3(g)The equilibrium constant, K, has the value 1.121054at a particular... Problem 52QAP: . For the reaction N2O4(g)2NO(g)the equilibrium constant K has the value 8.1103at a particular... Problem 53QAP: . Explain how the dissolving of an ionic solute in water represents an equilibrium process. Problem 54QAP: . What is the special name given to the equilibrium constant for the dissolving of an ionic solute... Problem 55QAP: . Why does the amount of excess solid solute present in a solution not affect the amount of solute... Problem 56QAP: . Which of the following will affect the total amount of solute that can dissolve in a given amount... Problem 57QAP: . Write the balanced chemical equation describing the dissolving of each of the following sparingly... Problem 58QAP: . Write the balanced chemical equation describing the dissolving of each of the following sparingly... Problem 59QAP: . K for copper(II)hydroxide, Cu(OH)2, has a value 2.21020at 25 °C. Calculate the solubility of... Problem 60QAP Problem 61QAP: . A saturated solution of nickel(II) sulfide contains approximately 3.6104gof dissolved NiS per... Problem 62QAP: . Most hydroxides are not very soluble in water. For example, for nickel(II) hydroxide. Ni(OH)2, is... Problem 63QAP: . The solubility product constant, Ksp, for calcium carbonate at room temperature is approximately... Problem 64QAP: . Calcium sulfate, CaSO4, is only soluble in water to the extent of approximately 2.05 g/L at 25 °C.... Problem 65QAP: . Approximately 1.5103of iron(II) hydroxide. Fe(OH)2(s), dissolves per liter of water at 18 °C.... Problem 66QAP: . Chromiurn(III) hydroxide dissolves in water only to the extent of 8.21105M at 25 °C. Calculate... Problem 67QAP: . Magnesium fluoride dissolves in water to the extent of 8.0102g/L at 25°C. Calculate the solubility... Problem 68QAP: . Lead(II) chloride, PbCl2(s), dissolves in water to the extent of approximately 3.6102Mat 20 °C.... Problem 69QAP: . Mercury(I) chloride, Hg2Cl2, was formerly administered orally as a purgative. Although we usually... Problem 70QAP: . The solubility product of iron(III) hydroxide is very small: Ksp=41038at 25 °C. A classical method... Problem 71AP: . Before two molecules can react, chemists envision that the molecules must first collide with one... Problem 72AP: . Why does an increase in temperature favor an increase in the speed of a reaction? Problem 73AP: . The minimum energy required for molecules to react with each other is called the _______ energy. Problem 74AP: . A(n) _______ speeds up a reaction without being consumed. Problem 75AP: . Equilibrium may be defined as the ________ of two processes, one of which is the opposite of the... Problem 76AP: . When a chemical system has reached equilibrium, the concentrations of all reactants and products... Problem 77AP: . What does it mean to say that all chemical reactions are, to one extent or another, reversible? Problem 78AP: . What does it mean to say that chemical equilibrium is a dynamic process? Problem 79AP: . At the point of chemical equilibrium, the rate of the forward reaction _________ the rate of the... Problem 80AP: . Equilibria involving reactants or products in more than one state are said to be _________ . Problem 81AP: . According to Le Châtelier’s principle, when a large excess of a gaseous reactant is added to a... Problem 82AP: . Addition of an inert substance (one that does not participate in the reaction) does not change the... Problem 83AP: . When the volume of a vessel containing a gaseous equilibrium system is decreased, the _________ of... Problem 84AP: . Consider the following reaction at some temperature: H2O(g)+CO(g)H2(g)+CO2(g)K=2.0 Some molecules... Problem 85AP: . What is meant by the solubility product for a sparingly soluble salt? Choose a sparingly soluble... Problem 86AP: . For a given reaction at a given temperature, the special ratio of products to reactants defined by... Problem 87AP: . Many sugars undergo a process called mutarotation, in which the sugar molecules interconvert... Problem 88AP: . Suppose K=4.5103at a certain temperature for the reaction PCl5(g)PCl3(g)+Cl2(g)If it is found that... Problem 89AP: . For the reaction CaCO3(s)CaO(s)+CO2(g)the equilibrium constant K has the form K=[CO2]. Using a... Problem 90AP Problem 91AP: . Teeth and bones are composed, to a first approximation, of calcium phosphate. Ca3(PO4)2(s). The K... Problem 92AP: . Under what circumstances can we compare the solubilities of two salts by directly comparing the... Problem 93AP: . How does the collision model account for the fact that a react ion proceeds faster when the... Problem 94AP Problem 95AP: . Explain why the development of a vapor pressure above a liquid in a closed container represents an... Problem 96AP Problem 97AP Problem 98AP: . For the reaction N2(g)+3Cl2(g)2NCl3(g)an analysis of an equilibrium mixture at a particular... Problem 99AP: . Gaseous phosphorus pentachloride decomposes according to the reaction PCl5(g)PCl3(g)+Cl2(g)The... Problem 100AP: . Write the equilibrium expression for each of the following heterogeneous equilibria. a.... Problem 101AP: . Write the equilibrium expression for each of the following heterogeneous equilibria. a.... Problem 102AP: . Consider the following generic reaction: 2A2B(g)2A2(g)+B2(g) Some molecules of A2B are placed in a... Problem 103AP Problem 104AP: . The reaction PCl3(l)+Cl2(g)PCl5(s)liberates 124 kJ of energy per mole of PCl3reacted. Will an... Problem 105AP Problem 106AP: . For the reaction N2(g)+3H2(g)2NH3(g)K=1.3102at a given temperature. If the system at equilibrium... Problem 107AP: . The equilibrium constant for the reaction 2NOCl(g)2NO(g)+Cl2(g)has the value 9.2106at a particular... Problem 108AP Problem 109AP Problem 110AP Problem 111AP: . Mercuric sulphide, HgS, is one of the least soluble salts known, with Ksp=1.61054at 25 °C.... Problem 112AP Problem 113AP: . For the reaction N2(g)+3H2(g)2NH3(g), list the types of bonds that must be broken and the type of... Problem 114AP: . What does the activation energy for a reaction represent? How is the activation energy related to... Problem 115AP Problem 116AP Problem 117AP Problem 118AP Problem 119AP Problem 120CP Problem 121CP: . Suppose that for a hypothetical reaction: A2(g)+2B(g)2AB(g)It is determined that at a certain... Problem 122CP Problem 123CP: . The reaction H2(g)+I2(g)2HI(g)has Kp=45.9at 763 K. A particular equilibrium mixture at 763 K... Problem 124CP Problem 125CP Problem 126CP: . Consider the following exothermic reaction at equilibrium: N2(g)+3H2(g)2NH3(g)Predict how the... Problem 1CR Problem 2CR Problem 3CR Problem 4CR: How is the strength of an acid related to the position of its ionization equilibrium? Write the... Problem 5CR Problem 6CR: How is the pH scale defined? What range of pH values corresponds to acidic solutions? What range... Problem 7CR: 7. Describe a buffered solution. Give three examples of buffered solutions. For each of your... Problem 8CR Problem 9CR Problem 10CR: . Explain what it means that a reaction has reached a state of chemical equilibrium. Explain why... Problem 11CR: . Describe how we write the equilibrium expression for a reaction. Give three examples of balanced... Problem 12CR Problem 13CR Problem 14CR: . In your own words, paraphrase Le Châtelier’s principle. Give an example (including a balanced... Problem 15CR Problem 16CR Problem 17CR: a. Write the conjugate base for each of the following Brensted- Lowry acids. Oj, H2SOj. HC1O4. NH/.... Problem 18CR: . Identify the Brønsted-Lowry conjugate acid-base pairs in each of the following. a.... Problem 19CR Problem 20CR Problem 21CR Problem 22CR Problem 23CR Problem 24CR: . The solubility product of magnesium carbonate, MgCO3, has the value Ksp=6.82106at 25 °C. How many... format_list_bulleted


 Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning




