
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
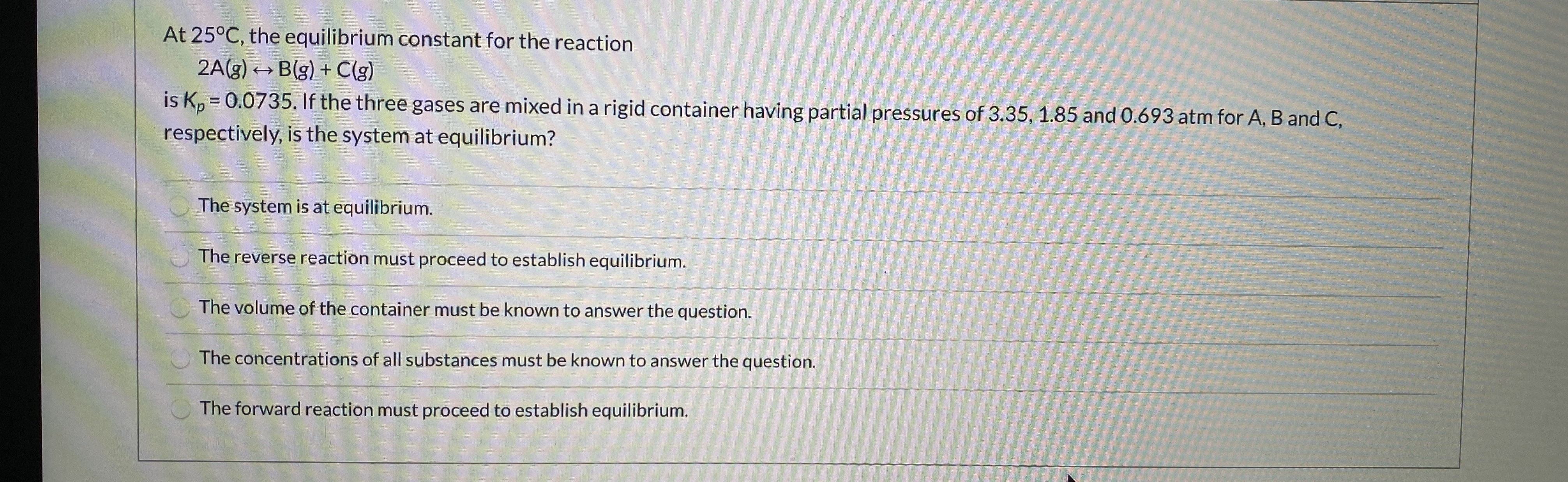
Transcribed Image Text:At 25°C, the equilibrium constant for the reaction
Blg) + Clg)
2A(3)
is K,-0.0735.If the three gases are mixed in a rigid container having partial pressuros of 3.35, 185 and 0.693 atm for A, B and C,
respectively, is the system at equilibrium?
The system is at equilibrium.
The reverse reaction must proceed to establish equilibrium.
The volume of the container must be known to answer the question.
The concentrations of all substances must be known to answer the question.
The forward reaction must praceed to establish equilibrium,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The equilibrium constant Ke for the reaction PC13 (g) + Cl2 (g) PCI5 (g) equals 2.7 at 330°C. a A sample of 38.4 g of PČl; is placed in a 2.3 L reaction vessel and heated to 330°C. What are the equilibrium concentrations of all of the species? [PCI5] = M [PCI3] = | [Cl2] =arrow_forwardPlease don't provide handwritten solution .....arrow_forwardA chemical engineer is studying the following reaction: N(g)+3 H,(9) - 2 NH;(g) At the temperature the engineer picks, the equilibrium constant K, for this reaction is 0.0016. The engineer charges ("fills") three reaction vessels with nitrogen and hydrogen, and lets the reaction begin. He then measures the composition of the mixture inside each vessel from time to time. His first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time he measures the compositions. reaction compound pressure expected change in pressure vessel 11.39 atm Of increase O I decrease O (no change) H. 25.28 atm O t increase OI decrease O (no change) A NH, 16.95 atm O t increase OI decrease O (no change) 12.82 atm O t increase OI decrease O (no change) 28.45 atm O f increase OI decrease O (no change) B NH, 21.46 atm O f increase O I decrease O (no change) N 10.92 atm O f increase OI decrease O (no change) O I decrease O (no change) H. 23.86…arrow_forward
- Br2(g) + Cl2(g) 2BRCI(g) the equilibrium concentrations were found to be [Br,) - 3.5 x 103 M, [CI,] = 1.2 x 10 2 M, and [BrCl] = 1.4 x 10 2 M. Write the equilibrium expression, and calculate the equilibrium constant for this reaction at this temperature. K = < Prev 9 of 27 Nexarrow_forwardSteam reforming of methane ( CH, ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 2.0 L flask with 3.8 atm of methane gas and 2.0 atm of water vapor, and when the mixture has come to equilibrium measures the partial pressure of hydrogen gas to be 3.0 atm. Calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant digits. K, 0 x10 ?arrow_forwardSulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. An industrial chemist studying this reaction fills a 5.0 L flask with 4.3 atm of sulfur dioxide gas and 3.9 atm of oxygen gas, and when the mixture has come to equilibrium measures the partial pressure of sulfur trioxide gas to be 3.9 atm. Calculate the pressure equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. Round your answer to 2 significant digits. K, = 0arrow_forward
- The equilibrium constant, Kc, for the following reaction is 6.30 at 723 K. 2NH3 (9) ⇒ N₂ (9) + 3H₂ (9) If an equilibrium mixture of the three gases in a 12.8 L container at 723 K contains 0.496 mol of NH3(g) and 0.448 mol of N₂, the equilibrium concentration of H₂ is M.arrow_forwarddont provide handwriting solution ....arrow_forwardThe equilibrium constant K, is 24 x 10¹ at a certain temperature for the reaction: 2NO() N₂(g) + O(g) Refer to the following table obtained under a set of experimental conditions T 2 3 Porf aben) 0.010 0.0078 0.0062 0.11 0.36 0.51 1.0 0.76 0.18 (i) For which of the above experiments is the system at equilibrium? (ii) Calculate K for the reaction. (6) For those that are not at equilibrium, in which direction will the system shift?arrow_forward
- The equilibrium constant, K, for the following reaction is 1.80x104 at 298 K. NH,HS(s)=NH3(g)+ H2S(g) Calculate the equilibrium concentration of H2S when 0.221 moles of NH,HS(s) are introduced into a 1.00 L vessel at 298 K.arrow_forwardPlease don't provide handwritten solution .....arrow_forwardMethanol liquid burns readily in air. One way to represent this equilibrium is: 2 CH3ОН(1) + 3 02(9)+ 2 CO2(g) + 4 H20(g) We could also write this reaction three other ways, listed below. The equilibrium constants for all of the reactions are related. Write the equilibrium constant for each new reaction in terms of K, the equilibrium constant for the reaction above. 1) CНH3ОН(1) + 3/2 02(9)+ =cO2(g) + 2 H20(g) K1 = %D 2) 2 CO2(9) + 4 H20(g) 2 CH3ОН (1) + 3 02(9) K2 = 3) СO2(g) + 2 H20(g) еснзон (1) + 3/2 02(g) K3 : %3D Drag and drop your selection from the following list to complete the answer: к1/2 1/K (1/K)!/2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY