
Understanding Business
12th Edition
ISBN: 9781259929434
Author: William Nickels
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Fast solve accurate please
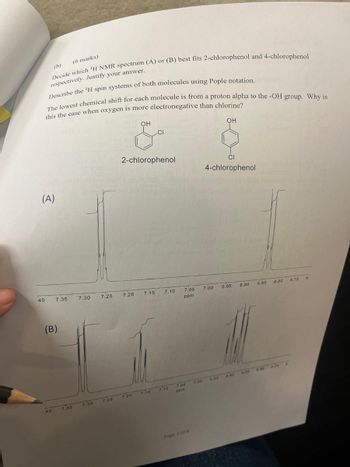
Transcribed Image Text:(b)
(6 marks)
Decide which 'H NMR spectrum (A) or (B) best fits 2-chlorophenol and 4-chlorophenol
respectively. Justify your answer.
Describe the 'H spin systems of both molecules using Pople notation.
The lowest chemical shift for each molecule is from a proton alpha to the -OH group. Why is
this the case when oxygen is more electronegative than chlorine?
(A)
OH
CI
OH
2-chlorophenol
CI
4-chlorophenol
40
7.35
7.30
7.25
7.20
7.15
7.10
7.05
7.00
6.95
6.90 6.85
6.80
6.75
6.
ppm
(B)
40
7.35
7.30
7.25 7.20
7.15
7.10
7.05
ppm
7.00
6.78
Page 3 of 8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Q1 (Process Analysis) - An MSU startup is contemplating a new process to refurbish and recycle rundown equipments. The five steps to the process are: Step Description 1 Register for the process 2 Strip off bad parts 3 Scrub and clean parts 4 Insert new parts 5 Polish and deliver Time Required per equipment 10.0 minutes 15.0 minutes 8.0 minutes 10.0 minutes 12.0 minutes. One employee will be assigned to each step. Employees will work a 40-hour week (no breaks) and rotate jobs each week. City of Lansing requires 200 refurbished equipments per week but they are unsure that the MSU startup can deliver. a. Create a column chart that displays the cycle time for each of the 5 steps. b. What is the processing rate for each step, in Equipments/Hour? Step 1 2 3 Processing Rate (equipments/hour) 6 4 7.5 4 5 6 5 c. What is the bottleneck? I believe that the bottleneck is Step 2 as that step takes the longest. d. Assuming the process runs as designed, what is the maximum weekly output? Maximum…arrow_forwardWhat are th benefits of having a paperless law office? Are many office's converting to paperless offices? Would it be worth the time and money spent to convert to a paperless law office? Does the size of the law firm matter if they should convert or not?arrow_forwardCan someone please help solve.arrow_forward
- Problem 7-13 In an initial survey designed to estimate the percentage of time air-express cargo loaders are idle, an analyst found that loaders were idle in 5 of the 45 observations. a. What is the estimated percentage of idle time? (Round your answer to 2 decimal places. Omit the "%" sign in your response.) Estimated percentage of idle time % b. Based on the initial results, approximately how many observations would you require to estimate the actual percentage of idle time to within 4 percent with a confidence of 95 percent? (Round your z value to 2 decimal places. Do not round any other Intermediate calculations. Round UP your final answer to the next whole number.) Number of observationsarrow_forwardWhat are the advantages and limitations of using Little’s Law?arrow_forwardWhat are some possible disadvantages of moving people to new jobs every 3 to 5 years?arrow_forward
- 1arrow_forwardPlease do not give solution in image format thankuarrow_forwardA fast-food restaurant has a drive-thru window and during peak lunch times can handle a maximum of 60 cars per hour with one person taking orders, assembling them, and acting as cashier. The average sale per order is $9.50. A proposal has been made to add two workers and divide the tasks among the three. One will take orders, the second will assemble them, and the third will act as a cashier. With this system, it is estimated that 85 cars per hour can be serviced.arrow_forward
- 14 Meetings are considered time wasters for managers. Specify one solution to control this time waster. * 5 Enter your answerarrow_forwardWhat is the total cost for these services ?arrow_forwardABC Solutions is a traditional company in a textile industry with a combined workforce of 150 staff. Due to increased levels of competition, this company is now increasingly finding it difficult to compete – especially as the larger companies seem to be a little more innovative, agile and responsive to client requirements. Management have noticed that staff, especially the highly skilled ones, are now leaving the company to seek work elsewhere (in these larger companies). This has meant appointing new staff and undertaking a considerable amount of re-training to replace those that have left the company. Apart from impacting on core business operations, ABC Solutions has started witnessing a drop in its profit margin. Given this position, senior management at ABC Solutions have decided to implement a performance measurement tool (PMT) that would allow them to align the strategic, management and operational requirements of the business. They are therefore considering the adoption of the…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Understanding BusinessManagementISBN:9781259929434Author:William NickelsPublisher:McGraw-Hill Education
Understanding BusinessManagementISBN:9781259929434Author:William NickelsPublisher:McGraw-Hill Education Management (14th Edition)ManagementISBN:9780134527604Author:Stephen P. Robbins, Mary A. CoulterPublisher:PEARSON
Management (14th Edition)ManagementISBN:9780134527604Author:Stephen P. Robbins, Mary A. CoulterPublisher:PEARSON Spreadsheet Modeling & Decision Analysis: A Pract...ManagementISBN:9781305947412Author:Cliff RagsdalePublisher:Cengage Learning
Spreadsheet Modeling & Decision Analysis: A Pract...ManagementISBN:9781305947412Author:Cliff RagsdalePublisher:Cengage Learning Management Information Systems: Managing The Digi...ManagementISBN:9780135191798Author:Kenneth C. Laudon, Jane P. LaudonPublisher:PEARSON
Management Information Systems: Managing The Digi...ManagementISBN:9780135191798Author:Kenneth C. Laudon, Jane P. LaudonPublisher:PEARSON Business Essentials (12th Edition) (What's New in...ManagementISBN:9780134728391Author:Ronald J. Ebert, Ricky W. GriffinPublisher:PEARSON
Business Essentials (12th Edition) (What's New in...ManagementISBN:9780134728391Author:Ronald J. Ebert, Ricky W. GriffinPublisher:PEARSON Fundamentals of Management (10th Edition)ManagementISBN:9780134237473Author:Stephen P. Robbins, Mary A. Coulter, David A. De CenzoPublisher:PEARSON
Fundamentals of Management (10th Edition)ManagementISBN:9780134237473Author:Stephen P. Robbins, Mary A. Coulter, David A. De CenzoPublisher:PEARSON

Understanding Business
Management
ISBN:9781259929434
Author:William Nickels
Publisher:McGraw-Hill Education

Management (14th Edition)
Management
ISBN:9780134527604
Author:Stephen P. Robbins, Mary A. Coulter
Publisher:PEARSON

Spreadsheet Modeling & Decision Analysis: A Pract...
Management
ISBN:9781305947412
Author:Cliff Ragsdale
Publisher:Cengage Learning

Management Information Systems: Managing The Digi...
Management
ISBN:9780135191798
Author:Kenneth C. Laudon, Jane P. Laudon
Publisher:PEARSON

Business Essentials (12th Edition) (What's New in...
Management
ISBN:9780134728391
Author:Ronald J. Ebert, Ricky W. Griffin
Publisher:PEARSON

Fundamentals of Management (10th Edition)
Management
ISBN:9780134237473
Author:Stephen P. Robbins, Mary A. Coulter, David A. De Cenzo
Publisher:PEARSON