
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Determine the product of the reaction.
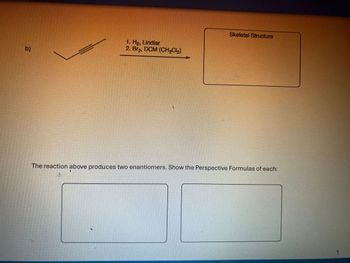
Transcribed Image Text:b)
1. Ho, Lindlar
2. Br₂, DCM (CH₂C₁₂)
Skeletal Structure
The reaction above produces two enantiomers. Show the Perspective Formulas of each:
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 2. Use the Newman projection to identify the structure shown: (a) as a Fischer projection (b) as a bond-line structure (c) by name Circle your answer for each part. CH2CH3 F+ H CH(CH3)2 CH2CH3 H+ F CH(CH3)2 三F H CH2CH3 H H F no CH(CH3)2 geslaan sam o Y (S)-4-fluoro-2-methylhexane F (R)-4-fluoro-2-methylhexanearrow_forwardH3C --CH3 Which structure(s) below represent(s) the diastereoisomer(s) of the compound on the picture? Br CH3 C CH3 A B CH3 H3C 'Br Br CH3 H3C Br CH3 E CH3 --CH3 H3C.. Br Br Br H3C -.arrow_forward3) Draw the ECSF structures of (CH3)3CO (tert-butoxide ion) and CH30º (methoxide ion). Why is (tert-butoxide a PN but methoxide a GN even though both have a full O? (As it turns out, both are SBs) Redraw (CH3)3coe (ECSF) Why PN? Redraw CH30S (ECSF) Why GN?arrow_forward
- Determine the product of the reactionarrow_forwardOH If the above molecule is molecule A, and is in a 50/50 mixture with molecule B, the mixture would be deemed racemic. Draw molecule B. What is the relationship between molecule A and B?arrow_forward(a) Classify each of the following pairs of structures as constitutional isomers, conformational isomers, configurational isomers or identical. Br and Br Br NH, CH, HO но H,C. ii. and CH, CH, Vi. and он Ph CH, он CH, Ph iv. andarrow_forward
- 4. Explain the following result- what strange regioselectivity is observed and why? (hint, what will always happen in the presence of a strong acid?): HNO3 HOAC NO2 O2N HNO3 H2SO4 NO2arrow_forward1. (a) Provide a name for each molecule (I narrowed it down to three subparts)arrow_forwardI need an explanation on this problem.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
