
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
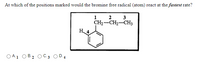
Transcribed Image Text:At which of the positions marked would the bromine free radical (atom) react at the fastest rate?
1
2
3
CH2 --CH2-CH3
H 4.
O A.1 O B.2 O C.3 O D.4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- OD OB O A O C Which of the following is an energy diagram for a two-step reaction? kJ/mol free e reaction coordinate A mm k reaction coordinate reaction coordinate B. reaction coordinate C. D.arrow_forward4. Consider the reaction shown below. Which term best describes this reaction? a. Addition b. Elimination c. Rearrangement d. Substitution + Br₂ Br Brarrow_forward← A → C FI 4 app.aktiv.com @ F2 (CH3CH₂)ǝN OH 80 F3 3 S PP Br U 000 000 F4 in FS S Problem 23 of 55 MacBook Air F6 g F7 A B с D E 3 C NaH DMF NaCl DMF NaHCO3 DMF NaH H₂O PP. (CH3CH₂)2N DII F8 O Done DD FO PP S PS A F10 P 4 8 AX B U F11 ★ + 0 Submit (1)) ⠀ F12arrow_forward
- What islare) the predicted productis) of the reaction shown? I. KOH 2. CH,CH,CH,CH,Br 3. H,0 но. он NH2 'NH2 NH2 OH II NH2 NH2 OH IV NH NH2 NH 一 Oarrow_forwardC1. Subject :- Chemistryarrow_forward25 идё массам 1) Tsci 2) NaI S excess CH₂ MI Felly/Al₂O3 (ene reaction) CH₂D AIM CI (Prins Fan) E B COM X Naci DMSD 150°C how is this formed?arrow_forward
- 4. Choose the compound that could be a product of this Heck reaction. (D represents isotope ²H, deuterium.) H Oll + D Pd(PPH3)2 H 0 ? A B C go po po H D E Farrow_forwardPredict the product. Br. Br so, H2SO, Br HO3S Br Br Br SO3H Br. Br Br SO3 Br Br Brarrow_forwardWhich product is likely NOT to form in the reaction in Figure 10-9? OA B ОС ODarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY