
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
please answer the step 8
Transcribed Image Text:At the beginning of the compression process of an air standard Otto cycle, p1 = 1 bar, T1= 300 K. The maximum temperature in the
cycle is 2250 Kand the compression ratio is 9.8. The engine has 4 cylinders and an engine displacement of Va = 2.5 L. Determine per
cylinder:
a) the volume at state 1.
b) the air mass per cycle.
c) the heat addition per cycle, in kJ.
d) the heat rejection per cycle, in kJ.
e) the net work per cycle, in kJ.
f) the thermal efficiency.
g) the mean effective pressure, in bar.
h) Develop a full exergy accounting per cycle, in kJ. Let To = 300 K, po=1 bar.
i) Devise and evaluate the exergetic efficiency for the cycle.
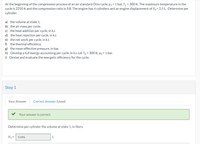
Step 1
Your Answer
Correct Answer (Used)
Your answer is correct.
Determine per cylinder the volume at state 1, in liters.
V1= 0.696
L
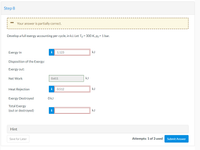
Transcribed Image Text:Step 8
Your answer is partially correct.
Develop a full exergy accounting per cycle, in kJ. Let To = 300 K, po = 1 bar.
Exergy In
i
1.123
kJ
Disposition of the Exergy:
Exergy out:
Net Work
0.611
kJ
Heat Rejection
i
0.512
kJ
Exergy Destroyed
OkJ
Total Exergy
(out or destroyed)
i
Hint
Save for Later
Attempts: 1 of 3 used
Submit Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- I am attaching both questions for 4 and 5 with the question in the image. thank you. NOTE : So the last person answered this question WITHOUT refencing the answer for whether question 4 or 5 answeres were given, so i am asking for question 5(or the answer for the question that was NOT solved because it was not referenced.) These were the following answers given to me from the last person on bartleby who answered my question without referencing whether it was the answer for question 4 or 5. 1 pass 2 fail 3 fail 4 passarrow_forwardDraw SFD and BMDarrow_forwardWhich one of the following medals has the lowest density? tungsten aluminum magnesium titaniumarrow_forward
- The attached answer was provided for the attached question. The equation uses the given temperature in celcius and the answer is also given in celcius. The temperature was used in the equation without converting from F and the answer was supposed to be in F. I am worried that even if I converted the answer to celcius that it would be correct because temperature was used in the equation using the incorrect unit.arrow_forwardPls do stepwise and correct.arrow_forwardProblem-AA: Consider the following system of equations: -123 = 1.5a + 25n 5 = -21a +n Calculate the two unknows.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY