
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
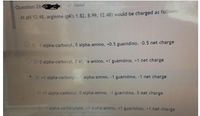
Transcribed Image Text:Saved
Question 264
At pH 12.48, arginine (pK's 1.82, 8.99, 12.48) would be charged as follows:
O 1) -1 alpha-carboxyl, 0 alpha-amino, +0.5 guanidino, -0.5 net charge
O 2) 0 alpha-carboxyl, 0 alpha-amino, +1 guanidino, +1 net charge
3) +1 alpha-carboxyl, -1 alpha-amino, -1 guanidino, -1 net charge
O4) +1 alpha-carboxyl, 0 alpha-amino, -1 guanidino, O net charge
5) 1 alpha-carboxylate, +1 alpha-amino, +1 guanidino, +1 net charge
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- The pH vs charge graph for a triprotic amino acid is shown below. Please answer the following questions about the amino acid. Net Charge 0 2 4 Alpha amino [Select] 6 R [Select] pH a. Which of the following triprotic amino acids is this? Alpha carboxyl [Select] 8 10 12 [ Select] 14 1 0.5 0 -0.5 -1 b. What is the isoelectric point? (Please select the appropriate range in which the isoelectric point falls) (Hint - look up the pKa values in the pKa table for amino acids, which was in video one of today's lecture) [Select] -1.5 c. What form of this amino acid dominates at a pH of 14? Please select the correct form for each ionizable group. -2arrow_forwardStereochemistry in biological molecules is often denoted by the D- and L- convention, instead of the R- and S- configurations determined by the Cahn-Ingold-Prelog methodology. Naturally occurring α-amino acids are typically in the L-configuration. The Fischer projection of L-alanine is shown. L-alanine H₂N- H3C- HOOC Identify which structures are equivalent to the L-configuration. H COOH H CH3 NH₂ -COOH NH₂ CH3 -H CH3 COOH H -NH₂ H₂N- H CH3 COOH NH₂ H CH3 -COOHarrow_forwardThe E. coli enzyme triose phosphate isomerase has a pI of 5.7. What would the charge on this enzyme be below a pH of 5.7? What about above? What would the charge be at a pH of 5.7?arrow_forward
- What is the principal form of arginine at pH 8.0? Approximately what fraction is in this form?arrow_forwardWhich of the twenty naturally occurring amino acid side chains are charged at a pan of 1.00? pH of 7.00? pH of 12.00? Make a table.arrow_forwardTitration of alanine by a strong base, for example NaOH, reveals two pk's. Which is the titration reaction occurring at pK1 (pK1 = %3D 2.34)? O-NH2 + COH →-NH + H2O O-COOH + OH →-CO + H20 -COOH + -NH2 → -COO" + -NH2+ -CoO +-NH2* → -COOH +-NH2 O -NH3* + OH →-NH2 + H2Oarrow_forward
- A) Refer to the figure below, Identify and explain the two types of reactions, and describe what are the importance of these reactions to our bodies. Reaction 1 H Monomer Reaction 2 H H OH + H H H₂O H H₂O OH + H H OH OH + H H₂O H₂O OH OH + H OH OH OH OH B) What are the main chemical interactions that determine and maintain the quaternary structure of proteins. Also, what are the conditions that can alter these interactions? [arrow_forwardThis amino acid is: (Note that the non-ionized form is shown; remember that backbone groups are irrelevant to classification) a) Polarized (+) charged b) Nonpolar c) Polar and (-) charged d) Polar unchargedarrow_forwardΦ and ψ in the Ramachandran plot (below) are: a) Rotational angles around the bond between the α-carbon and N-H (Φ) and C=O (ψ). b) Amino acid solubility in octanol (Φ) and water (ψ). c) Hydrogen bond angles in α-helices (Φ) and β-sheets (ψ). d) Amino acid solubility in water (Φ) and octanol (ψ).arrow_forward
- The carbon-nitrogen peptide bond is rigid, but rotation can take place about the N-Ca and the Ca-C bonds in a protein (Ca is the a carbon atom). These bond angles define the conformation of the peptide chain. Consider the Ramachandran plot to answer both questions. Which dihedral angles and residues would not likely occur in a ß sheet? 180 120 60 -60 -120 -180 -180 180 + (degrees) OA. 0=-51°; U=+153°; many Gly and Pro residues D=+60°; u=+60°; many Gly residues В. OC. D=-57°; W=-49°; many Ala residues D=-139°; W=+135°; many Val residues OD. D=-120°; U=+120°; many Tyr residues DE. (səəbap)arrow_forwardThe pka of the side chain of histidine is 6.00. What is the percent of protonated histidine at pH 6.4? 15.3% 28.5% 45.3% O 66.6%arrow_forwardCompare the stability of the two salt bridges shown circled in blue at pH 8.4. The carboxylate ions are completely de- protonated at pH 8.4 The pka of the amino group (upper left) is 8.1 and the pka of the amino group (bottom right) is 8.7. (d) NH, F-8- Ed (b) O-H- M5 CH₂COOH (c) 5 (b) CH, CH. H--O=C (d) -H H CH,OH (b) KCHJ NH, (a) The bottom right salt bridge is stronger due to the partial positive charge of +2/3. The bottom right salt bridge is stronger due to the partial positive charge of +1/3. The upper left salt bridge is stronger due to the partial positive charge of +1/3. The upper left salt bridge is stronger due to the partial positive charge of +2/3.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON