
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
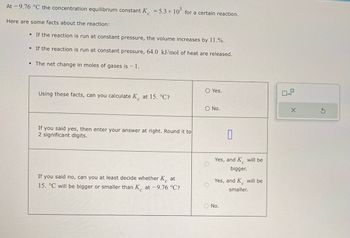
Transcribed Image Text:At -9.76 °C the concentration equilibrium constant K = 5.3 × 105 for a certain reaction.
Here are some facts about the reaction:
• If the reaction is run at constant pressure, the volume increases by 11.%.
• If the reaction is run at constant pressure, 64.0 kJ/mol of heat are released.
• The net change in moles of gases is - 1.
Using these facts, can you calculate K at 15. °C?
If you said yes, then enter your answer at right. Round it to
2 significant digits.
If you said no, can you at least decide whether K at
15. °C will be bigger or smaller than K at -9.76 °C?
O Yes.
O No.
0
Yes, and K will be
bigger.
Yes, and K will be
smaller.
O No.
0
x10
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A reaction is studied at various temperatures, and a graph of ln K ( equilibrium constants on y axis) versus 1/T (Temperature on x axis) is prepared in Excel. The equation of the line is found to be y = - 9500 x + 21.5. What is the ΔSfor this reaction in J/mol/K? (assume R=8.314Jmol⋅K)arrow_forwardConsider the following equilibrium: N2 (g)+3H2(g)2NH3 (g) AG = -34. kJ Now suppose a reaction vessel is filled with 2.15 atm of nitrogen (N2) and 2.19 atm of ammonia (NH3) at 236. °C. Answer the following questions about this system: OO rise ☐ x10 fall OO Under these conditions, will the pressure of NH3 tend to rise or fall? Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of NH3 will tend to rise, can that be changed to a tendency to fall by adding H2? Similarly, if you said the pressure of NH3 will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H2 needed to reverse it. Round your answer to 2 significant digits. yes no ☐ atm Sarrow_forwardWhat would happen to the (i) equilibrium constant, K, of the following reactions and the (ii) direction of equilibrium shift upon heating?arrow_forward
- Ammonia (NH3) decomposes to form nitrogen gas and hydrogen gas with an enthalpy of 89.4kJ/mol. Aclosed container is charged with 6.0 atm ammonia and heated to 25.0°C. At equilibrium the pressure of H2 is3.60atm. a. Write the balanced reaction.arrow_forwardFor the reaction below, what is the effect of raising the temperature? 4A(g) + B(g) 3C(g) AH = - 405 kJ Equilibrium shifts to the right; the reaction makes more products. O The reaction makes more of both products and reactants, so equilibrium is unaffected O Equilibrium re-establishes Equilibrium shifts to the left; the reaction makes more reactants.arrow_forwardConsider the reaction: PbCl2(s) → Pb2*(aq) + 2 Cl (aq) AH° = 23.30 kJ/mol and AS° = -12.5 J/K-mol a) When solid PbCl2 is dissolved in water at 25°C, what are the concentrations of Pb2* and Cl at equilibrium? (Hint: Do your ICE chart and Law of Mass Action.) Concentration of Pb2+: mol/L and Concentration of Cl = mol/L (Do not use superscripts, subscripts, or carets. Write 1.2 x 10-3 as 1.2x10-3 or 1.2e-3.) b) At 25°C, This reaction is (reactant or product) favored. Justify your answer with the appropriate calculation on the separate sheet of paper. c) The Gibbs free energy at 25°C, when both the concentrations of the lead ion and chloride ion are 1.2 x 10-³M is kJ/mol. Show your calculations on the separate sheet of paper.arrow_forward
- Please answer ALL parts of the question and show your work. Consider this reaction: 2H2S(g) + 3O2(g) <-----> 2H2O(g) + 2SO2(g) H= -1036 kJ Predict whether the forward or reverse reaction will reestablish equilibrium is disturbed by (a) expanding the container (b) removing SO2 (c) rasing the temperature (d) water vapor is addedarrow_forwardConsider the following equilibrium: N₂ (g) + 3H₂(g)2NH₂ (g) AG = -34. KJ 2 Now suppose a reaction vessel is filled with 3.70 atm of hydrogen (H₂) and 8.46 atm of ammonia (NH3) at 1099. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of H ₂ tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding N₂? x Ś ? In other words, if you said the pressure of H₂ will tend to rise, can that be changed to a tendency to fall by adding N₂? Similarly, if you said the yes no pressure of H₂ will tend to fall, can that be changed to a tendency to rise 2 by adding N₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of N₂ needed to reverse it. atm Round your answer to 2 significant digits. ● Oarrow_forwardConsider the following system: 4 NH3(g) + 3 O2(g) -> 2 N2(g) + 6 H2O(l) AH = -1530.4 kJ (a) How will the amount of ammonia at equilibrium be affected by (1) removing O2(g)? (2) adding N,(g)? (3) adding water? (4) expanding the container at constant pressure? (5) increasing the temperature? (b) Which of the above factors will increase the value of K? Which will decrease it?arrow_forward
- Consider the system below at equilibrium at 200°C: 2C12 (g) + 2H20 (g) - 98.6KJ = 4HCI (g) + 02 (g) The change listed below would cause more reactants to form when equilibrium is re- established. Write A if true and B if false (Note: Answer is case sensitive) Decreasing the volume at constant temperaturearrow_forward9. What is the equilibrium constant for reaction below at 25 °C? (R=8.314 J/K-mol) 2 NO(g) + O2(g) 2 NO2(g) given AG° [NO(g)] =+86.6 kJ/mol and AG° [NO2(g)] =+51.2 kJ/mol. a. 3.9 x 10-13 b. 1.0 c. 2.6 x 1012 d. 1.6 x 106 е. 3.8 х 1028 of silver bromide is 5.4 x 10-13 at 298 K.arrow_forwardWhat is true about the following reaction at 25°C? H₂(g) + 2BrF(g) = 2HBr(g) + F₂(g) \Delta Hr\ deg = 115 kJ mol-¹ \Delta Sr\deg = 11.55 J mol-¹ K-¹ \Delta Gr\deg = 111 kJ mol-¹ i. The reaction is endothermic. ii. As the reaction proceeds heat is transferred from the surroundings to the system.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY