
Chemistry: An Atoms First Approach
2nd Edition
ISBN: 9781305079243
Author: Steven S. Zumdahl, Susan A. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
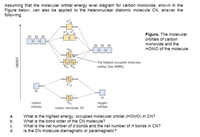
Transcribed Image Text:Assuming that the molecular orbital energy level diagram for carbon monoxide, shown in the
Figure below, can also be applied to the heteronuclear diatomic molecule CN, answer the
following:
2p
Figure. The molecular
orbitals of carbon
2р 2р 2р
monoxide and the
2р 2р 2р
HOMO of the molecule.
- The highest occupied molecular
orbital (the HOMO).
o 25
25
2s
carbon
orbitals
oxygen
orbitals
carbon monoxide, co
а.
b.
What is the highest energy, occupied molecular orbital (HOMO) in CN?
What is the bond order of the CN molecule?
What is the net number of o bonds and the net number of T bonds in CN?
c.
d.
Is the CN molecule diamagnetic or paramagnetic?
ENERGY
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain the difference between the and MOs for homonuclear diatomic molecules. How are bonding and antibonding orbitals different? Why are there two MOs and one MO? Why are the MOs degenerate?arrow_forwardThe photoelectron spectrum of HBr has two main groups of peaks. The first has ionization energy 11.88 eV. The next peak has ionization energy 15.2 eV, and it is followed by a long progression of peaks with higher ionization energies. Identify the molecular orbitals corresponding to these two groups of peaks.arrow_forwardThe B2 molecule is paramagnetic; show how this indicates that the energy ordering of the orbitals in this molecule is given by Figure 6.18a rather than 6.18b.arrow_forward
- 8) Consider the heteronuclear diatomic molecule, HCl with bonding orbital given by, citation legg W, = 0.205,H +0.98º3p,cI %D ght ventric FULL PRIC where ø is the (normalized) hydrogen 1s orbital and o,, c is the (normalized) chlorine BOG 3pz orbital. Assume that the atomic overlap integral is zero, | WisH W3p.cadt = 0. %3D all space a) Show that the bonding molecular orbital is normalized. Shot 232 PM BOO and B, b) What is the energy of the bonding orbital? Write your answer in terms of a,, ,a,,. T COS which are defined by: АМ Shbt 25PM ght ventric | V1s,H Hw cdt = | 4 1s,H RENT & || all space all space Che pulmc vein | Wis.H HySHdt =a,, and cdt = d3p. 1s, 3 Pz,CI right atriursen Shot 2021-02 5.56 PM pulmonary valve all space all space W étv A APR 4 29 260 Book A DD F11 F10arrow_forwardAn energy level scheme for the orbitals of second row diatomic molecules O, through Ne2 , lists the molecular orbitals in the following order of increasing energy 02 < o'2s < Ozp(z) < T2p(y)- 7T2P(x) < T*2p(y), 7T* 2p(x) < o*2plz) Based on this energy level scheme, how many electrons are there in all of the antibonding molecular orbitals of the F2* ion in its ground state?arrow_forwardDescribe the hybrid orbitals on the chlorine atom in theClO4-and ClO3- molecular ions. Sketch the expectedgeometries of these ions.arrow_forward
- The diagram that follows shows the highest-energy occupiedMOs of a neutral molecule CX, where element X is in thesame row of the periodic table as C. (a) Based on the numberof electrons, can you determine the identity of X? (b) Wouldthe molecule be diamagnetic or paramagnetic? (c) Considerthe p2p MOs of the molecule. Would you expect them to havea greater atomic orbital contribution from C, have a greateratomic orbital contribution from X, or be an equal mixtureof atomic orbitals from the two atoms?arrow_forwardFor the O2, O2+, O2-, Draw the molecular orbital electron configuration (1sσ2, 1sσ*2, 2sσ2, 2sσ*, 2pσ2, 2pπ2,,,, ) for O2-.arrow_forward. Both the simple VB model and the LCAO method predictthat the bond order of Be2 is 0. Explain how each arrivesat that conclusion.arrow_forward
- Draw a fully labelled molecular orbital energy level diagram for the molecule NP Phosphorus Nitride show only nitrogen 2p orbitals with ionisation energy of 2.33 x10-18J and phosphorus 3p orbitals ionisation energy 1.68x 10-18Jarrow_forwardAnsider he realhon N2 cg) +3 H2 (9)-)2NH, C9) If H.05 of N, realt lolth ese cess Hz, How many pames Of NHz will form.arrow_forwardFill in the Molecular Orbital Energy Diagram for the diatomic molecule 0₂. E 2p oxygenA 2s MO's 2p 17*2p П2Р | 02p 0*25 D 02s oxygenB In this case 02p 2s 2p < П2рarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning